Felbamate
General Information
Felbamate Impurities and Felbamate
Daicel Pharma offers high-quality Felbamate impurities, such as N-Aminocarbonyl Felbamate. It is vital for evaluating the quality, stability, and biological safety of Felbamate. In addition, Daicel Pharma specializes in the custom synthesis of Felbamate impurities and ensures their worldwide delivery.
Felbamate [CAS: 25451-15-4], an antiepileptic, is a bis(carbamate ester) of 2-phenylpropane-1,3-diol. Its properties are similar to other anticonvulsant compounds. Further, Felbamate manages Lennox-Gastaut syndrome and focal seizures. It inhibits the N-methyl-D-aspartate (NMDA) receptor glycine binding site and blocks excitatory amino acids effects, thus suppressing seizure activity.
Felbamate: Use and Commercial Availability
Felbamate is an anticonvulsant that treats patients with epilepsy. It treats partial seizures in adult patients. In addition, Felbamate is a neuroprotective agent that helps in preventing seizures. Felbamate reduces seizure spread and increases seizure threshold. Felbamate is available under the name Felbatol and other trade names.
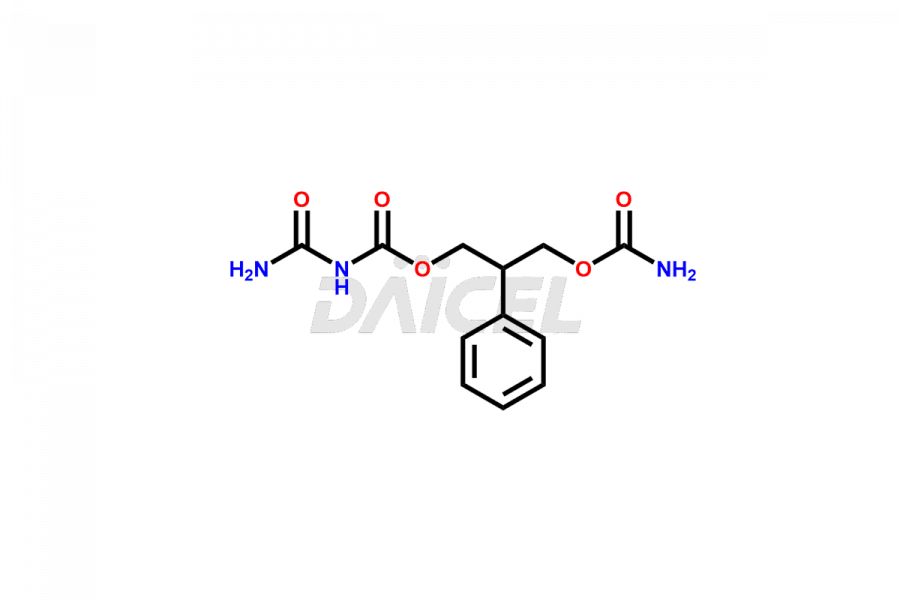
Felbamate Structure and Mechanism of Action

Felbamate has a chemical name, that is 2-Phenyl-1,3-propanediol dicarbamate. The chemical formula for Felbamate is C11H14N2O4 and its molecular weight is approximately 238.24 g/mol.
The mechanism of action for Felbamate is not clearly understood.
Felbamate Impurities and Synthesis
During the synthesis of Felbamate 1, impurities may form that may affect the safety and efficacy of the drug. These impurities form during the synthesis, storage, or degradation of Felbamate. Felbamate impurities need control and monitoring to improve the drug’s safety, efficacy, and storage.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Felbamate impurities, which includes N-Aminocarbonyl Felbamate. The CoA is from a cGMP-compliant analytical facility. It contains the complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2,3. We give additional data like 13C-DEPT and CHN on request. Daicel Pharma can prepare any unidentified Felbamate impurity or degradation product. In addition, Daicel Pharma offers highly purified isotope-labeled standards of Felbamate for bioanalytical research and BA/BE studies. We also provide a complete characterization report on delivery.
References
FAQ's
References
- 2-Phenyl-1,3-propanediol dicarbamate, GB798568, July 23, 1958, Carter Products, Inc.
- Romanyshyn, L. A.; Wichmann, J. K.; Kucharczyk, N.; Sofia, R. D., Simultaneous determination of felbamate and three metabolites in human plasma by high-performance liquid chromatography, Therapeutic Drug Monitoring, Volume: 16, Issue: 1, Pages: 83-9, 1994 DOI: (10.1097/00007691-199402000-00014)
- Hempenius, J.; Hendricks, G.; Hingstman, J.; Mensink, C. K.; Jonkman, J. H. G.; Lin, C. C., An automated analytical method for the determination of felbamate in human plasma by robotic sample preparation and reversed-phase high performance liquid chromatography, Journal of Pharmaceutical and Biomedical Analysis, Volume: 12, Issue: 11, Pages: 1443-51, 1994 DOI: (10.1016/0731-7085(94)00087-5)
Frequently Asked Questions
How do you detect and control impurities in Felbamate?
Manufacturers can test the starting materials according to regulatory specifications. In addition, they can monitor the process parameters to detect and control Felbamate impurities.
Why is it essential to control Felbamate impurities during synthesis?
Controlling Felbamate impurities during synthesis ensure drug safety, quality, and efficacy.
What are the side effects of Felbamate impurities?
Felbamate impurities can decrease the therapeutic effects, cause drug toxicity, and reduce product shelf-life.
How are Felbamate impurities characterized?
LC-MS analytical methods help characterize Felbamate impurities.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.