Finerenone
General Information
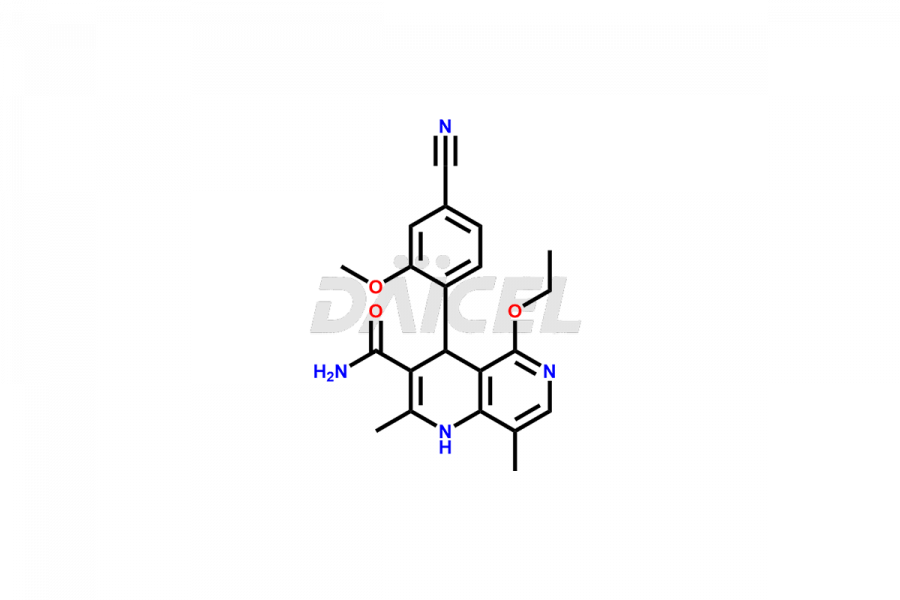
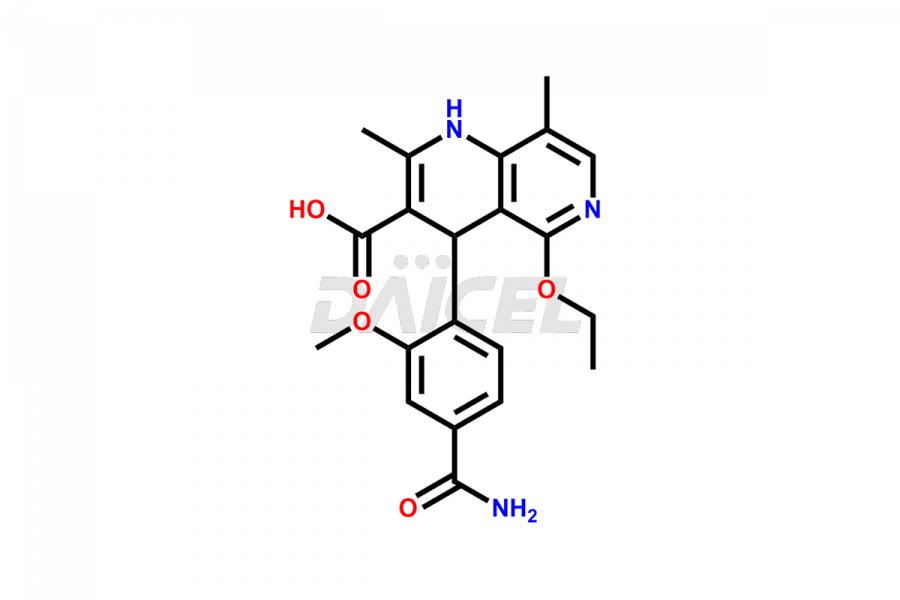
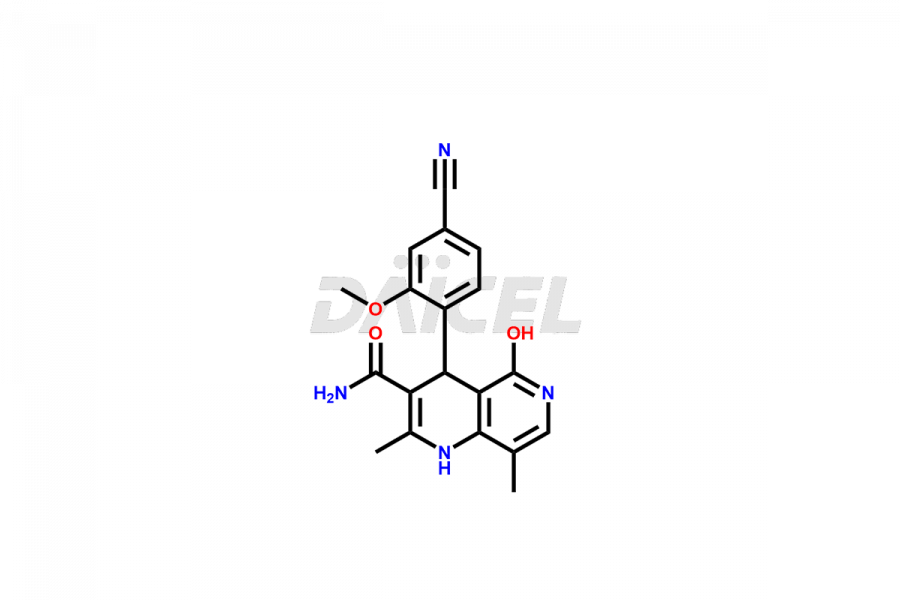
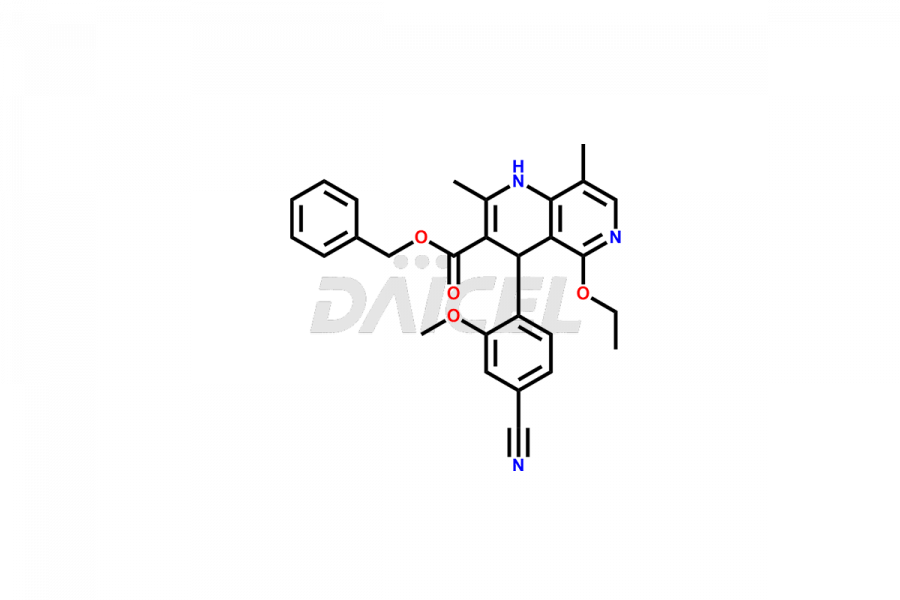
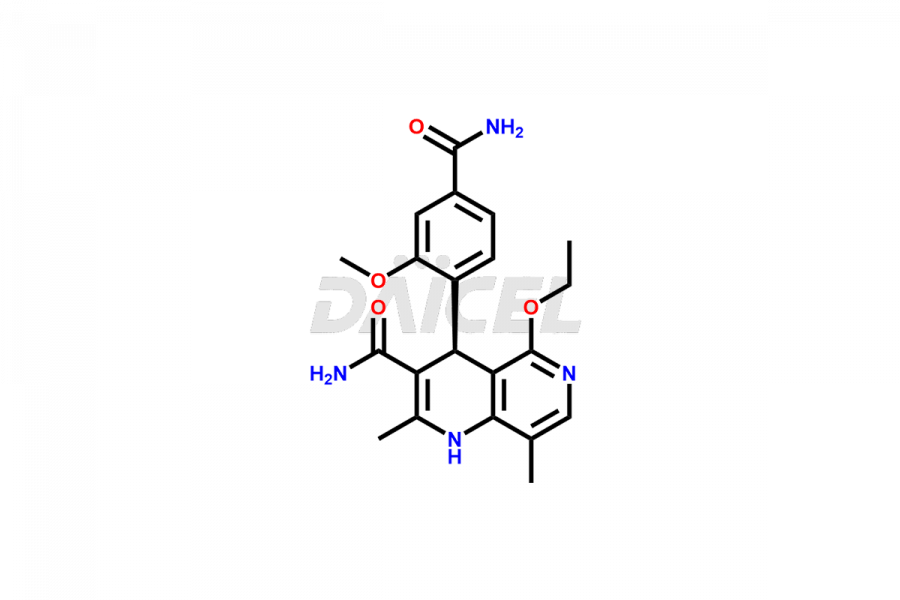
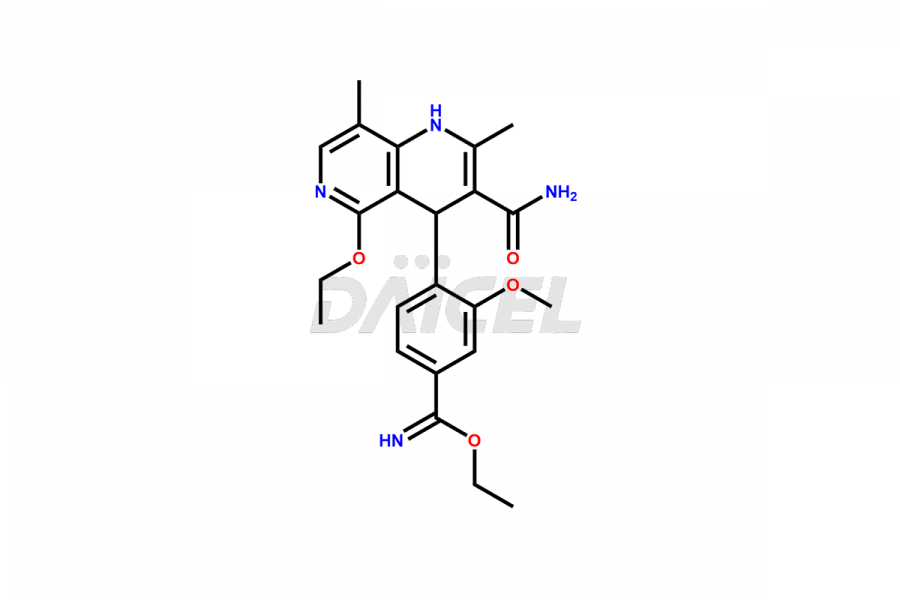
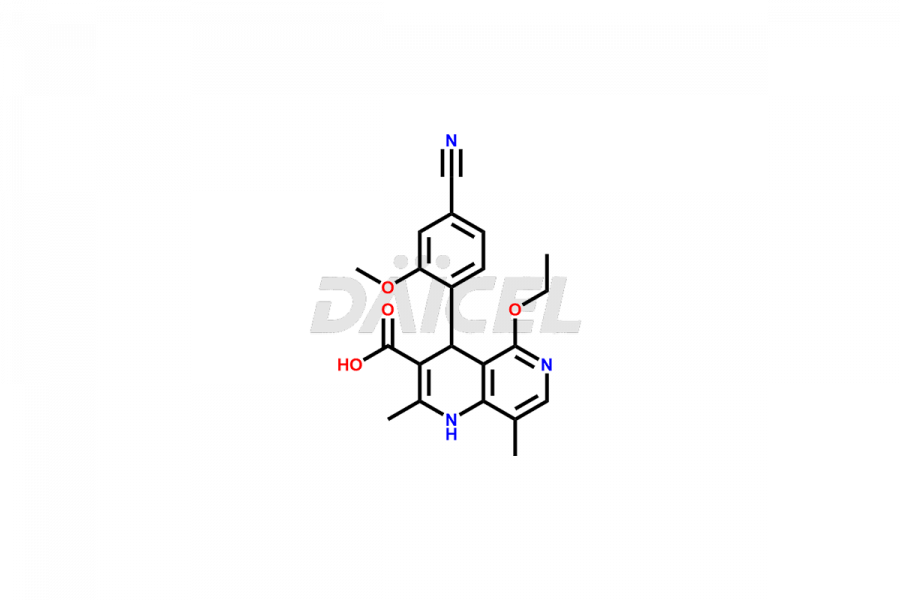
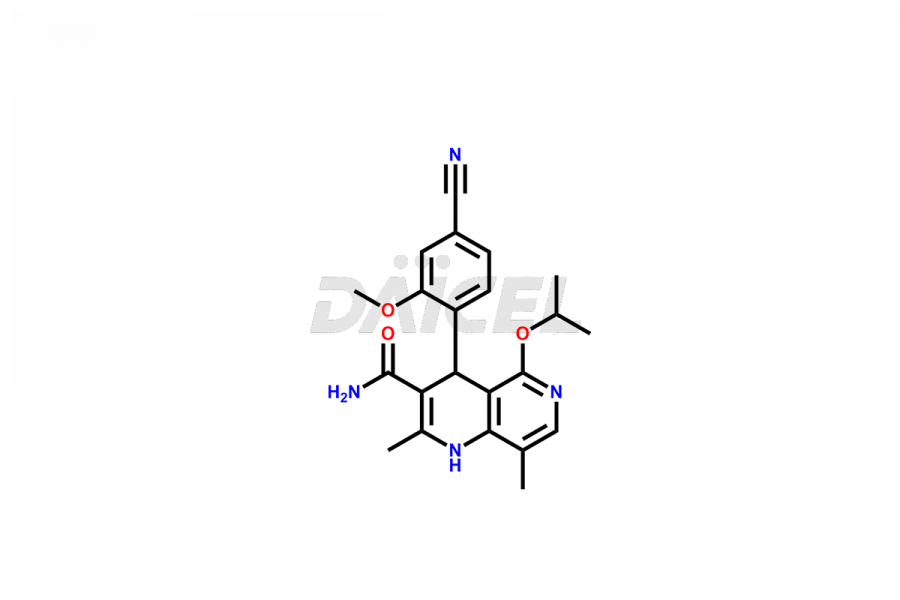
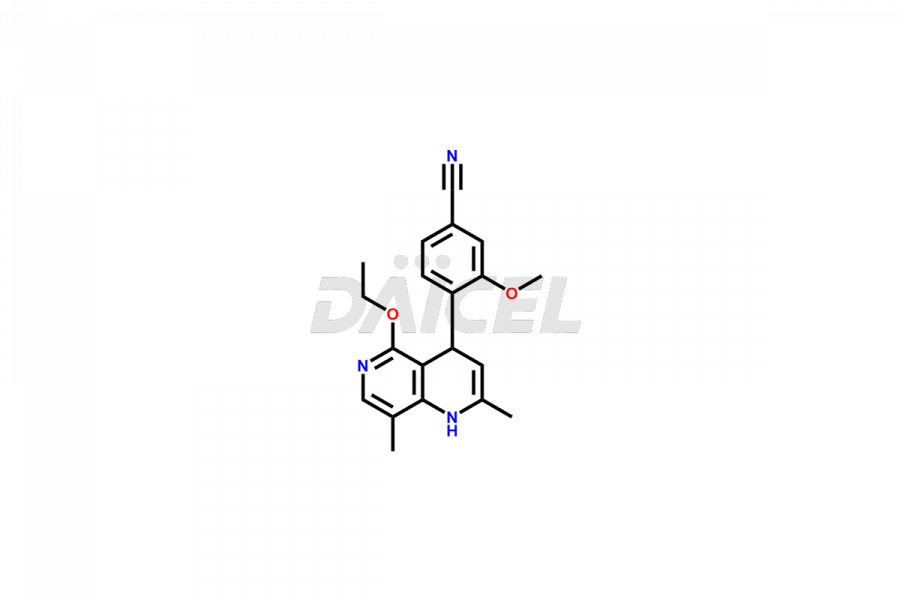
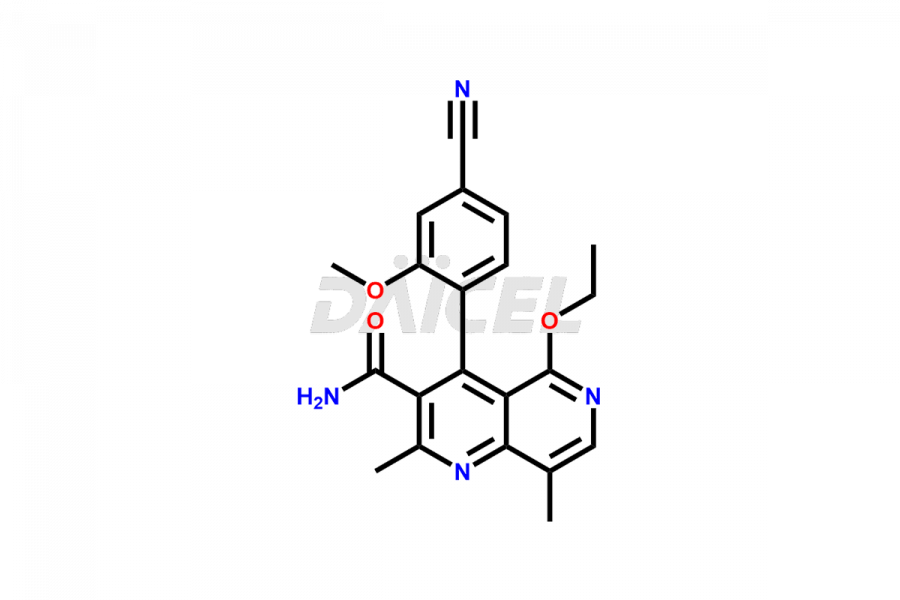
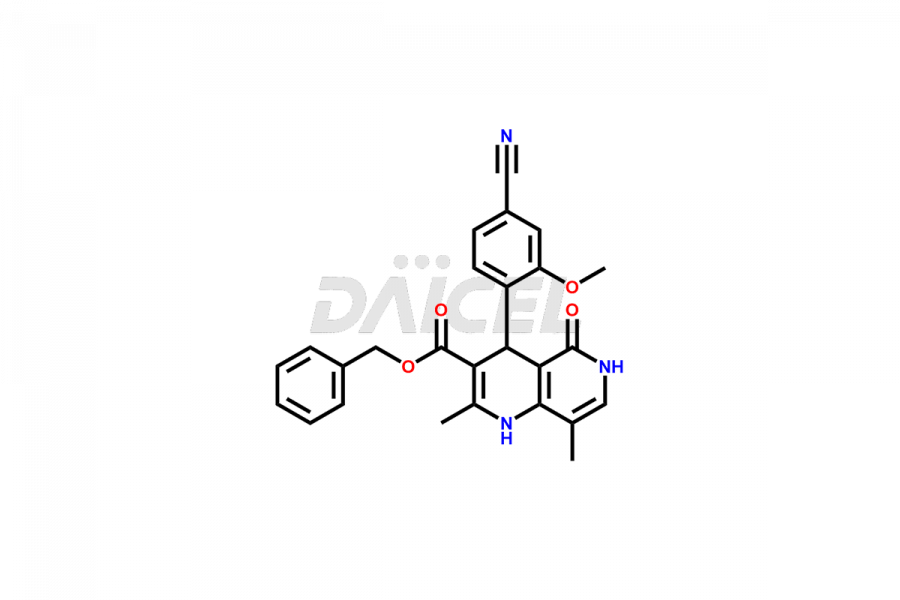
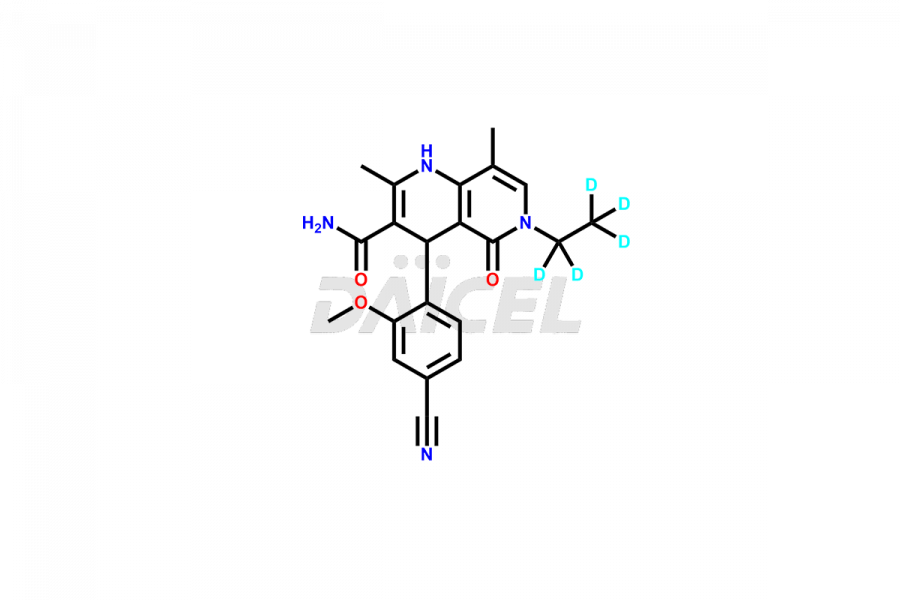
Finerenone Impurities and Finerenone
Daicel Pharma offers the best quality Finerenone impurities, such as Finerenone amide impurity, Finerenone Impurity 15, Finerenone Impurity 4, Finerenone M1, and Finerenone Related Impurity. It is vital for evaluating the quality, stability, and biological safety of Finerenone. In addition, Daicel Pharma specializes in the custom synthesis of Finerenone impurities and ensures their worldwide delivery.
Finerenone [CAS: 1050477-31-0] is a non-steroidal antagonist of the mineralocorticoid receptor (MR). It is a dihydropyridine channel blocker. It treats chronic kidney disease (CKD) associated with Type 2 diabetes (T2D) in adults. It reduces the risk of cardiovascular death, sustained eGFR decline, non-fatal myocardial infarction, and more in patients with CKD and T2D. Further, it treats patients with chronic kidney disease (stages 3 and 4 with albuminuria).
Finerenone: Use and Commercial Availability
Finerenone treats patients with diabetes and kidney disease. It inhibits mineralocorticoids like aldosterone, which regulates blood pressure and sodium retention. In addition, Finerenone lowers the occurrence of hyperkalemia. It helps in reducing hospitalization for heart failure in adult patients with chronic kidney disease with diabetes. Developed by Bayer Healthcare, Finerenone is available as oral tablets under the trade name Kerendia.
Finerenone Structure and Mechanism of Action


The chemical name of Finerenone is (4S)-4-(4-Cyano-2-methoxyphenyl)-5-ethoxy-1,4-dihydro-2,8-dimethyl-1,6-naphthyridine-3-carboxamide. The chemical formula for Finerenone is C21H22N4O3, and its molecular weight is approximately 378.43 g/mol.
Aldosterone and cortisol activate Finerenone, which regulates gene transcription. Finerenone blocks mineralocorticoid receptor (MR). It prevents sodium reabsorption and overaction in epithelial and nonepithelial tissues.
Finerenone Impurities and Synthesis
Impurities may form during the synthesis of Finerenone 1 that affect its safety, efficacy, and shelf-life. They form during the synthesis, storage, or degradation2 of Finerenone. It is necessary to control and monitor the impurities of Finerenone to improve the drug safety, efficacy, and storage.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Finerenone impurities, which includes Finerenone amide impurity, Finerenone Impurity 15, Finerenone Impurity 4, Finerenone M1, and Finerenone Related Impurity. The CoA is from a cGMP-compliant analytical facility. It contains the complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We give additional data like 13C-DEPT and CHN on request. Daicel Pharma can prepare any unidentified Finerenone impurity or degradation product. In addition, Daicel Pharma offers highly purified deuterium-labeled standard of Finerenone-D5 for bioanalytical research and BA/BE efficacy studies. We also provide a complete characterization report on delivery.
References
FAQ's
References
- Baerfacker, Lars; Kolkhof, Peter; Schlemmer, Karl-Heinz; Grosser, Rolf; Nitsche, Adam, Substituted 4-aryl-1,4-dihydro-1,6-naphthyridinamides and use thereof, EP2132206B1, April 1, 2015, Bayer Healthcare A.-G.Germany
- Arulselvan Murugesan and Annapurna Mukthinuthalapati Mathrusri, Forced Degradation Studies for Estimation of Finerenone by RP-HPLC Method, Acta Scientific Pharmaceutical Sciences (ISSN: 2581-5423), Volume 5 Issue 12 December 2021
Frequently Asked Questions
How do Finerenone impurities form?
By-products that appear during the manufacture or improper storage of Finerenone can lead to the formation of Finerenone impurities.
Which analytic method detects Finerenone impurities?
RP-HPLC analytical methods help detect Finerenone impurities.
What is the use of Finerenone impurities?
Finerenone impurities help in pharmaceutical research, method validation, and stability studies.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.