LOAD MORE
You're viewed 9 of 12 products
Daicel Pharma specializes in synthesizing impurities for Fluvoxamine, an active pharmaceutical ingredient. We offer impurities such as Fluvoxamine acid N-Acetyl Impurity, Fluvoxamine EP impurity I, Fluvoxamine EP impurity J, Fluvoxethanol, and Fluvoxketone, which play a vital role in evaluating the purity and safety of Fluvoxamine. Daicel Pharma also provides custom synthesis of Fluvoxamine impurities to meet specific client needs and offer worldwide delivery options.
Fluvoxamine [CAS: 54739-18-3] is an antidepressant, anxiolytic drug, and selective serotonin reuptake inhibitor (SSRI). It treats obsessive-compulsive disorder (OCD) and various anxiety disorders. Fluvoxamine is exerts its therapeutic effects by inhibiting the reuptake of serotonin, a neurotransmitter involved in regulating mood and anxiety. By increasing serotonin levels in the brain, Fluvoxamine helps alleviate symptoms associated with depression, OCD, and anxiety disorders.
Fluvoxamine, available under Luvox and Luvox CR, is an SSRI medication for treating OCD, social anxiety disorder, and major depression. Although it is less common than other SSRIs, it is highly effective in managing anxiety disorders. Furthermore, it treats bulimia nervosa.
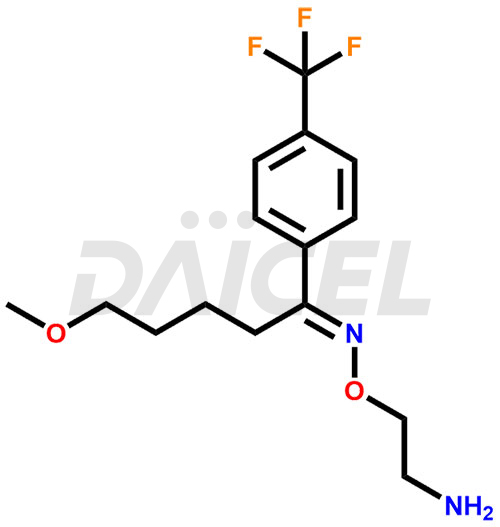
The chemical name of Fluvoxamine is (1E)- 5-methoxy-1-[4-(trifluoromethyl)phenyl]- O-(2-aminoethyl)oxime 1-Pentanone. Its chemical formula is C15H21F3N2O2, and its molecular weight is approximately 318.34 g/mol.
The mechanism of action of Fluvoxamine is due to serotonin reuptake inhibition in the brain neurons.
Fluvoxamine, an antidepressant and anxiolytic medication, may contain impurities that can from various sources. They occur during the manufacturing process1, degradation over time, or as residual solvents. Common Fluvoxamine impurities include related substances, degradation products, and residual solvents. They can affect the drug’s stability, potency, and overall quality. Therefore, strict quality control measures during the manufacturing process minimize impurity levels and ensure the purity of the final product.
Daicel Pharma, in adherence to cGMP standards, operates an analytical facility where we prepare Fluvoxamine impurity standards like Fluvoxamine acid N-Acetyl Impurity, Fluvoxamine EP impurity I, Fluvoxamine EP impurity J, Fluvoxethanol, and Fluvoxketone. We offer a comprehensive Certificate of Analysis (CoA) for these impurities, providing a detailed characterization report. The CoA includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. We can synthesize unknown Fluvoxamine impurities or degradation products. Each delivery has a comprehensive characterization report.
Yes, impurities in Fluvoxamine can change over time due to factors such as degradation, environmental conditions, or interactions with other substances. Stability studies evaluate the potential changes in impurity levels and ensure the quality and stability of the medication throughout its shelf life.
While efforts are ongoing to minimize impurities in Fluvoxamine, it is challenging to eliminate them. However, through rigorous quality control processes and adherence to regulatory guidelines, manufacturers strive to keep impurity levels within acceptable limits to ensure the safety and efficacy of the medication.
Methanol or acetonitrile are commonly used as solvents when analyzing many impurities in Fluvoxamine.
The recommendation is to store Fluvoxamine impurities at a controlled room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.