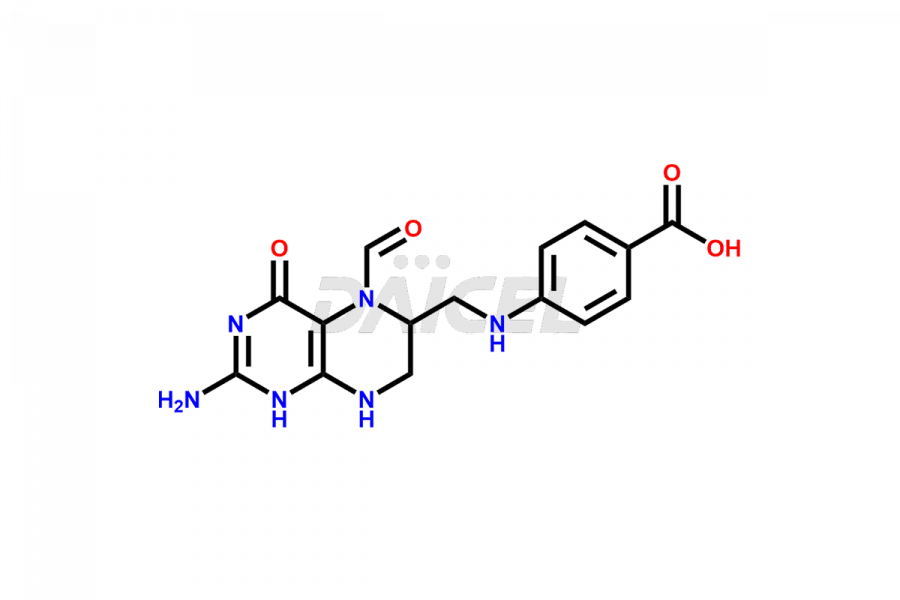
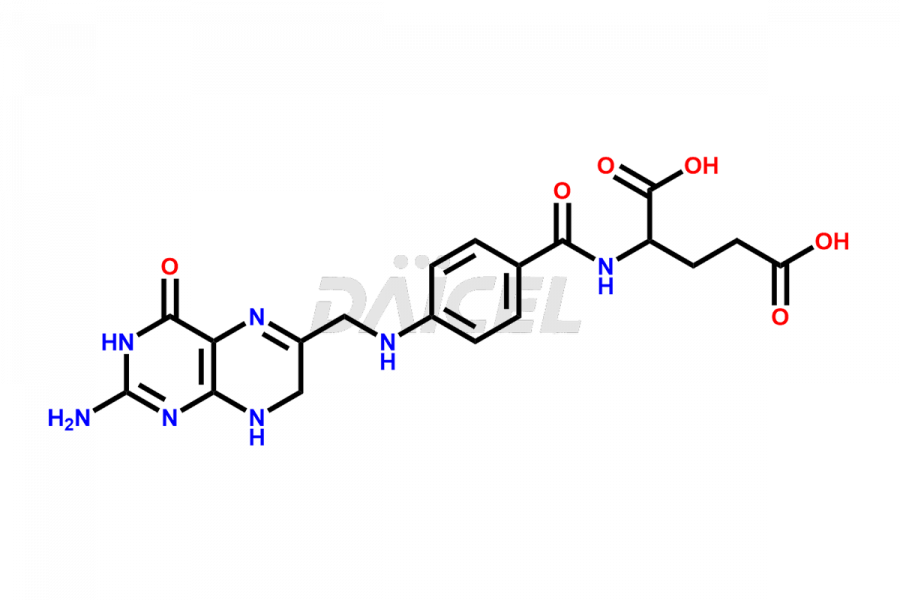
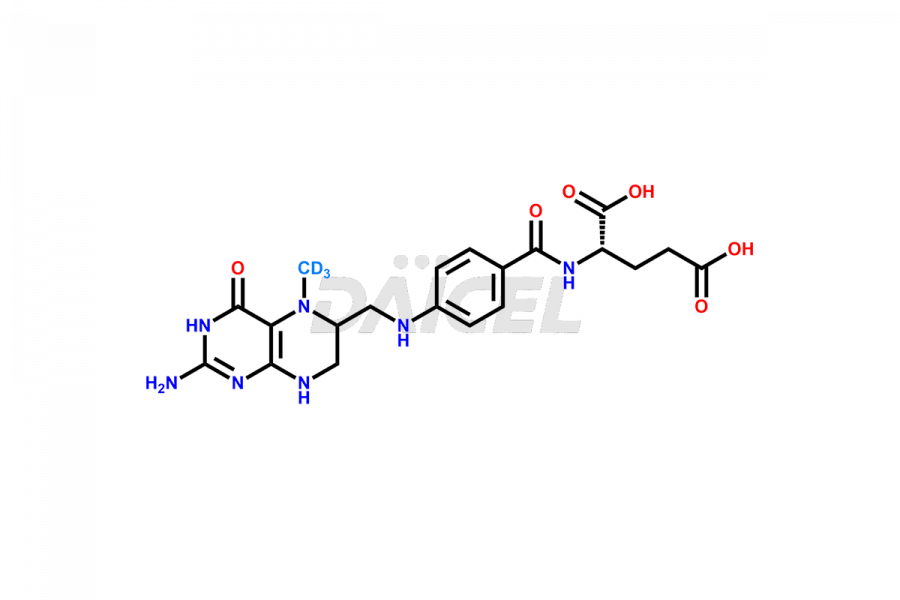
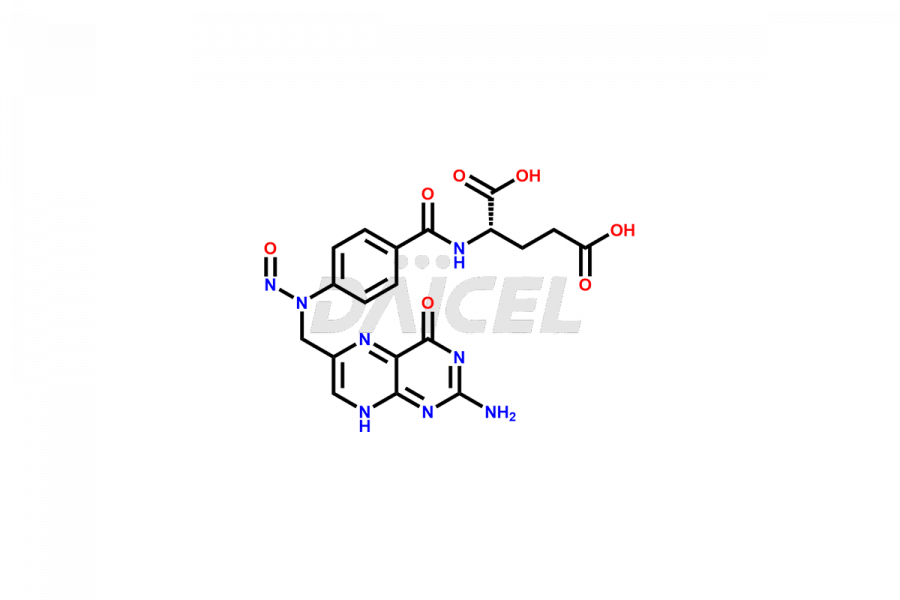
Folic acid
General Information
Folic acid Impurities and Folic acid
Daicel Pharma offers the best quality Folic acid impurities, such as 7,8-Dihydrofolicacid. It is vital for evaluating the quality, stability, and biological safety of Folic acid. In addition, Daicel Pharma specializes in the custom synthesis of Folic acid impurities and ensures their worldwide delivery.
Folic acid [CAS: 59-30-3] is Vitamin B-complex. It consists of a heterobicyclic pteridine ring. Folic acid has a synthetic form, folate. Some other names of Folic acid include Vitamin B9, Folacin, etc. It is available naturally in green plants. It is essential for hemoglobin formation and prevents anemia in humans. It is a water-soluble vitamin that treats megaloblastic anemia.
Folic acid: Use and Commercial Availability
Folic acid administration is via oral, subcutaneous, or intravenous methods. It prevents neural tube defects in babies during pregnancy. It is integral in the synthesis of thymine, a DNA component. Folic acid is necessary for amino acid and nucleotide synthesis. It acts as a nutritional supplement along with other vitamins. Its deficiency can cause various health ailments like anemia, cardiovascular diseases, cancer, inflammatory diseases, and more. Folic acid is available under different names by various manufacturers. Folicet, and Folvite are some of the trade names of Folic acid.
Folic acid Structure and Mechanism of Action


N-[4-[[(2-Amino-3,4-dihydro-4-oxo-6-pteridinyl)methyl]amino]benzoyl]-L-glutamic acid is the chemical name of Folic acid. The chemical formula for Folic acid is C19H19N7O6, and its molecular weight is approximately 441.40 g/mol.
Folic acid, as dihydrofolate (DHF), is converted to tetrahydrofolic acid (THF) due to dihydrofolate reductase enzyme activity. Further, tetrahydrofolic acid (THF) converts to 5-10-methylenetetrahydrofolate (5-10-MTHF) and DNA synthesis.
Folic acid Impurities and Synthesis
The synthesis of Folic acid1 may form impurities that affect drug safety, efficacy, and shelf-life. They form during the synthesis, storage, or degradation of Folic acid. It is necessary to control and monitor the impurities of Folic acid to prevent any inefficiencies in drug efficacy and safety.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Folic acid impurities, which includes 7,8-Dihydrofolicacid. The CoA is from a cGMP-compliant analytical facility. It contains the complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional data like 13C-DEPT and CHN on request. Daicel Pharma can prepare any unidentified Folic acid impurity or degradation product. In addition, Daicel Pharma offers highly purified isotope-labeled standards of Folic acid for bioanalytical research and BA/BE studies. We also provide a complete characterization report on delivery.
References
FAQ's
References
- Robert B. Angler et al. Synthesis of Pteroylglutamic Acid. III, J. Am. Chem. Soc. 1948, 70, 1, 25–26
- Williams, R. C.; Baker, D. R.; Schmit, J. A, Analysis of water-soluble vitamins by high-speed ion-exchange chromatography, Journal of Chromatographic Science, Volume: 11, Issue: 12, Pages: 618-24, 1973 DOI: (10.1093/chromsci/11.12.618)
Frequently Asked Questions
What are the different types of Folic acid impurities?
There are eight European Pharmacopeial impurities of Folic acid. In addition, two impurities occur due to photo-oxidation. Many known and unknown impurities of Folic acid are also present.
Which analytic methods detect Folic acid impurities?
Multidimensional NMR, FT-IR, UV-Vis, HRMS, and LC-MS are the analytical methods that help detect Folic acid impurities.
Why is it essential to isolate Folic acid impurities?
Isolation of Folic acid impurities helps in quality control and method validation during commercial production of Folic acid.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.