LOAD MORE
You're viewed 9 of 23 products
Daicel Pharma synthesizes more than twenty high-quality Formoterol impurities, including (R, R)-Formoterol, (S, R)-Formoterol, (S, S)-Formoterol, N-Benzyl Formoterol, rac-O-Benzyl N-Benzyl Formoterol, Formoterol (mixture of Diastereomers), Formoterol related compound I, etc., which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient, Formoterol. Moreover, Daicel Pharma offers custom synthesis of Formoterol impurities and delivers them globally.
Formoterol [CAS:73573-87-2] is a bronchodilator medicine that acts as a long-acting beta-2 adrenergic receptor agonist. It helps in the maintenance treatment of asthma and the prevention of bronchospasm.
Formoterol treats asthma and chronic obstructive pulmonary disease (COPD) in various formulations. For COPD treatment, Formoterol can be administered alone or in combination with long-acting muscarinic antagonists (LAMAs) or corticosteroids. For asthma treatment, Formoterol combines with mometasone furoate or budesonide for patients of different ages. Formoterol can act as a prophylaxis agent against exercise-induced bronchospasm. Formoterol is available under brand names, including Symbicort, Perforomist, Foradil, Dulera, Brovana, Breyna, etc.
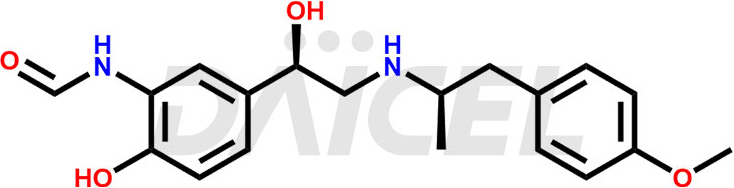
The chemical name of Formoterol is rel-N-[2-Hydroxy-5-[(1R)-1-hydroxy-2-[[(1R)-2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]formamide. Its chemical formula is C19H24N2O4, and its molecular weight is approximately 344.4 g/mol.
Formoterol selectively binds to beta-2 adrenergic receptors in the bronchial smooth muscles. It stimulates intracellular adenyl cyclase, an enzyme. Formoterol catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3′,5′-adenosine monophosphate (cAMP). Increased cAMP causes relaxation of the bronchial smooth muscles and reduces bronchospasms.
Formoterol impurities preparation help in the identification, quantification, and evaluation of the chemical properties and stability of the drug. It can also help in the development of analytical methods and regulatory compliance. The information obtained from the synthesis of impurities is crucial for drug safety, efficacy, and quality control. So, the synthesis1 of Formoterol impurities plays a significant role in pharmaceutical research and development.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for more than 20 Formoterol impurity standards, including (R, R)-Formoterol, (S, R)-Formoterol, (S, S)-Formoterol, N-Benzyl Formoterol, rac-O-Benzyl N-Benzyl Formoterol, Formoterol (mixture of Diastereomers), Formoterol related compound I, etc. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS2, and HPLC purity. We also provide 13C-DEPT and CHN on request. We give a complete characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Formoterol impurity or degradation product.
The purpose of synthesizing Formoterol impurities is to identify, characterize, and quantify the impurities present in Formoterol drug products. It assesses Formoterol's safety, efficacy, and quality while ensuring compliance with regulatory guidelines.
Formoterol impurities are typically detected and quantified using analytical methods such as high-performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC-MS).
The polar solvent methanol helps in analyzing Formoterol and its impurities.
Formoterol impurities should be stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.