Ganirelix
General Information
Ganirelix Impurities and Ganirelix
Daicel Pharma offers superior-quality Ganirelix impurities, such as D-Ser(4)-Ganirelix and L-Ala(10)Ganirelix. These impurities are essential for evaluating Ganirelix quality, stability, and biological safety. In addition, Daicel Pharma specializes in the custom synthesis of Ganirelix impurities and ensures their worldwide delivery.
Ganirelix [CAS: 124904-93-4] is a synthetic decapeptide developed by Syntex Research. It reduces gonadotropin secretion in patients undergoing assisted reproduction treatment. It is a drug to regulate luteinizing hormone responses in women undergoing fertility treatment.
Ganirelix: Use and Commercial Availability
Ganirelix prevents premature luteinizing hormone surges in women undergoing in vitro fertilization (IVF). It helps in the management of uterine fibroids, endometriosis, advanced prostate cancer, and benign prostatic hyperplasia. Many manufacturers prepare Ganirelix under brands like Antagon, Orgalutran, etc. The drug administration is through the subcutaneous route.
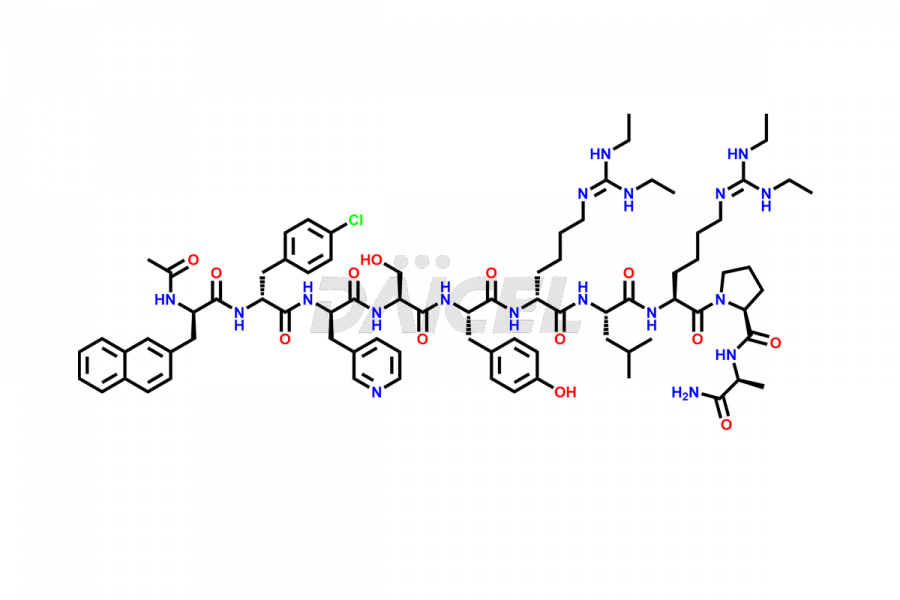
Ganirelix Structure and Mechanism of Action
The chemical name of Ganirelix is N-Acetyl-3-(2-naphthalenyl)-D-alanyl-4-chloro-D-phenylalanyl-3-(3-pyridinyl)-D-alanyl-L-seryl-L-tyrosyl-N6-[bis(ethylamino)methylene]-D-lysyl-L-leucyl-N6-[bis(ethylamino)methylene]-L-lysyl-L-prolyl-D-alaninamide. The chemical formula for Ganirelix is C80H113ClN18O13, and its molecular weight is approximately 1570.32 g/mol.
Ganirelix inhibits the gonadotropin-releasing hormone (GnRH) receptors in the pituitary gland. It prevents the release of hormones, such as luteinizing hormone (LH) and follicle-stimulating hormone (FSH).
Ganirelix Impurities and Synthesis
While synthesizing Ganirelix 1, impurities may form that will affect drug safety and efficacy. They form during the synthetic process, storage, or purification of Ganirelix. Manufacturers can control and monitor Ganirelix impurities to improve the drug’s safety, efficacy, and storage.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Ganirelix impurities, which includes D-Ser(4)-Ganirelix and L-Ala(10)Ganirelix. The CoA offered to clients is from a cGMP-compliant analytical facility, which has the complete characterization data2 such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We give additional spectral data on request. Daicel Pharma can prepare any unidentified Ganirelix impurity or degradation product. In addition, Daicel Pharma offers highly purified, stable isotope-labeled standards of Ganirelix. Daicel Pharma provides a complete characterization report accompanying the delivery.
References
FAQ's
References
- Nestor, John J., Jr.; McClure, Natalie L., Temporary minimal protection synthesis of serine-containing polypeptides, US5212288A, Feb 8, 1991, Syntex (U.S.A.), Inc., United States (https://patents.google.com/patent/US5212288A/en)
- Van Dorpe, Sylvia; Vergote, Valentijn; Pezeshki, Adel; Burvenich, Christian; Peremans, Kathelijne; De Spiegeleer, Bart, Hydrophilic interaction LC of peptides: columns comparison and clustering, Journal of Separation Science, Volume: 33, Issue: 6-7, Pages: 728-739, 2010 DOI: (10.1002/jssc.200900476)
Frequently Asked Questions
2.What causes the formation of Ganirelix impurities?
Side reactions and by-products formed during the synthetic process are the source of Ganirelix impurities.
3.What are the leachable impurities that form in the Ganirelix drug?
Acrylic acid leachable impurities form in the Ganirelix drug during storage.
4.Which analytical technique identifies polyacrylic acid-Ganirelix adduct leachable impurities?
Mass spectrometry identifies polyacrylic acid-Ganirelix adduct leachable impurities.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.