Glycocholic
General Information
Glycocholic acid Impurities and Glycocholic acid
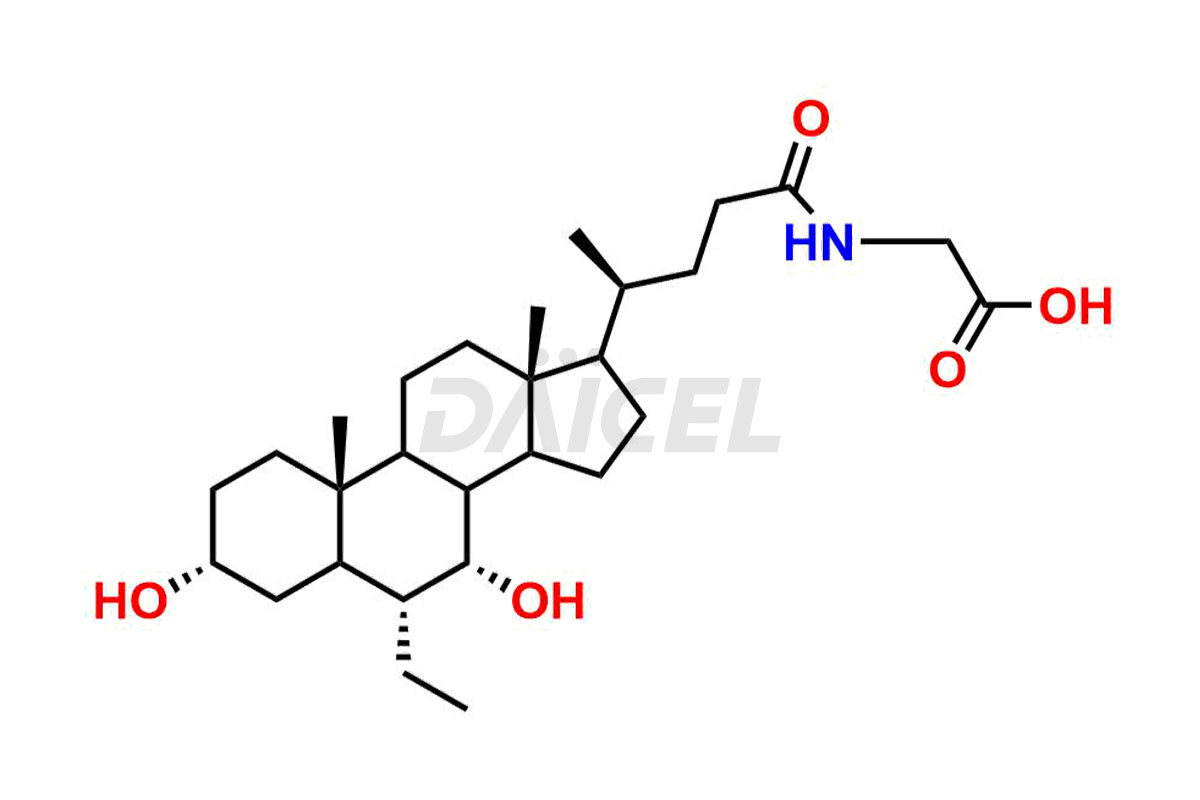
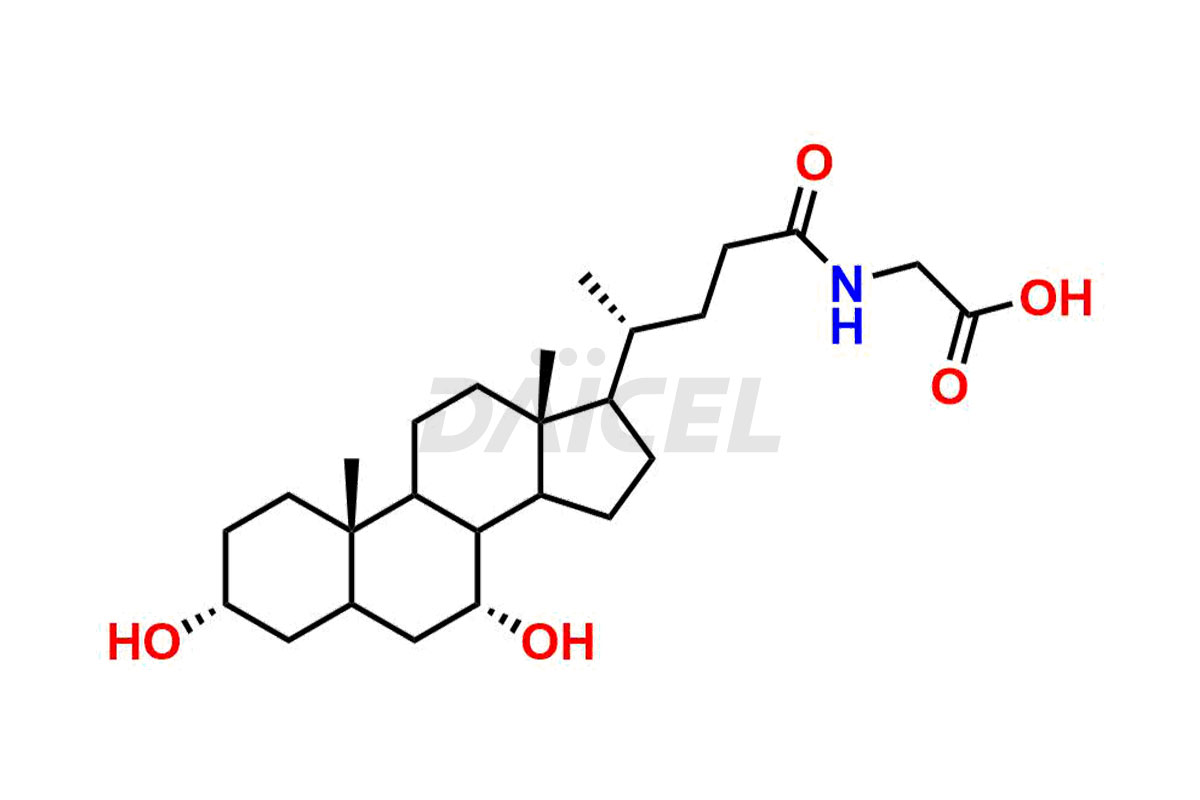
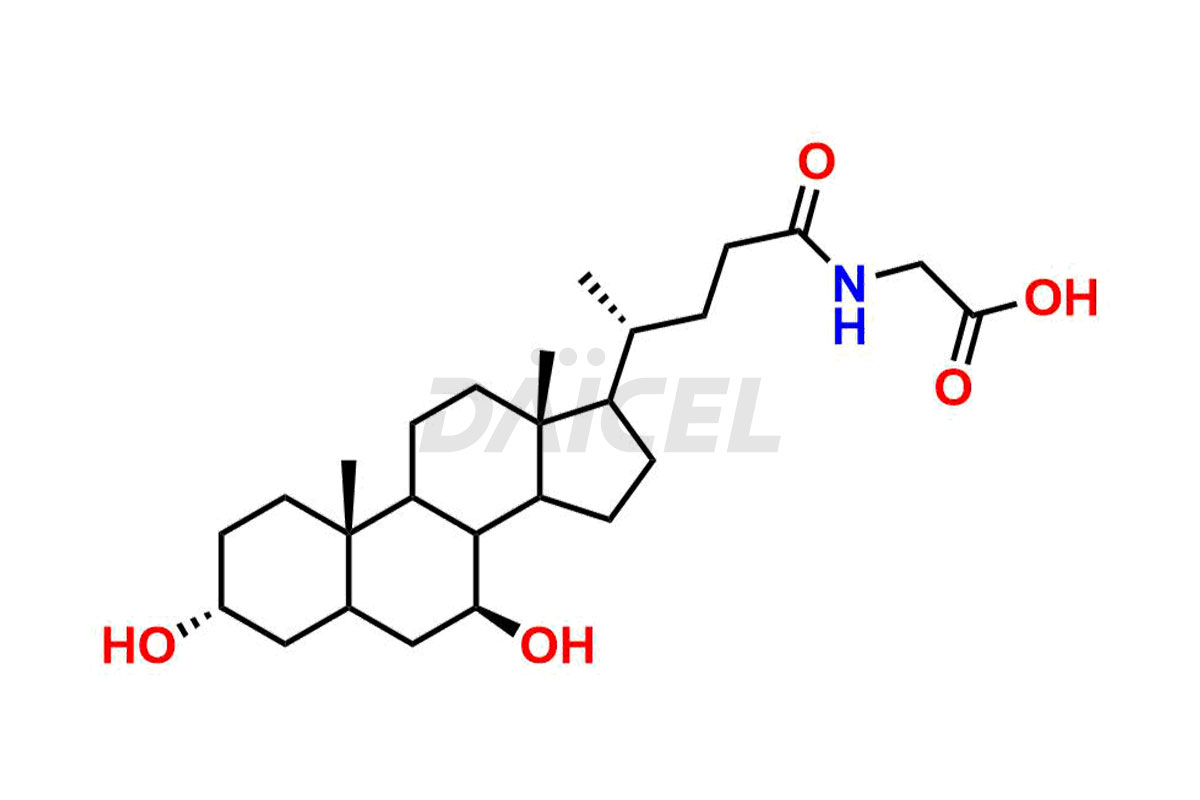
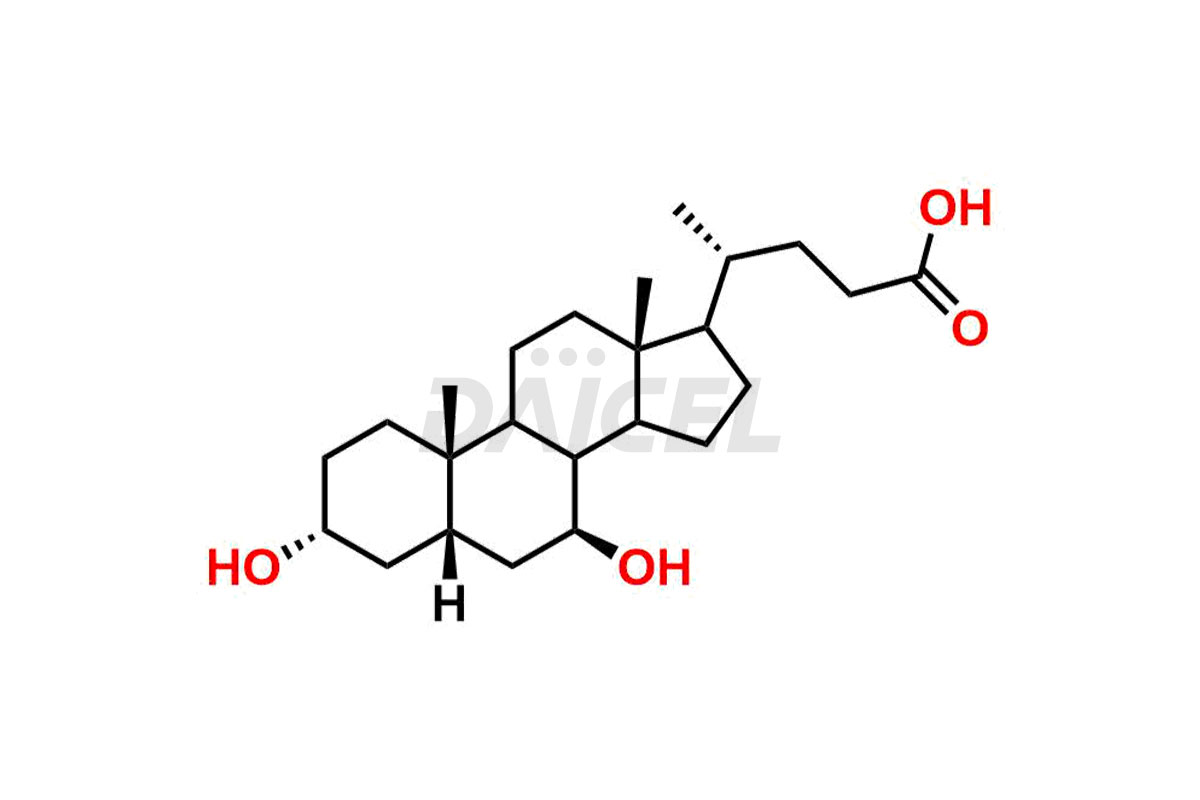
Daicel Pharma offers excellent-quality Glycocholic acid impurities, such as Glycine 6-ethylchenodeoxy cholate Impurity, Glycoursodeoxy cholic acid, Glycochenodeoxy cholic acid, and Ursochenodiol Impurity. They are vital for evaluating Glycocholic acid quality, stability, and biological safety. In addition, Daicel Pharma specializes in the custom synthesis of Glycocholic acid impurities and ensures worldwide delivery.
Glycocholic acid [CAS: 475-31-0] is a bile acid component of human bile. It is the glycine conjugate of cholic acid. In mammals, it is present as a sodium salt in the bile Cholic acid, a primary bile acid, combines with glycine to form Glycocholic acid through the N-acylase transferase process. Defects in this N-acylation process of cholic acid result in severe liver diseases diagnosed by Glycocholic acid.
Glycocholic acid: Use and Commercial Availability
Glycocholic acid emulsifies fats for absorption in the body. It is available as oral formation. It treats children and adolescents with inborn bile acid metabolism errors. Glycocholic acid helps in fat-soluble vitamin absorption and improves the growth of the affected children and adolescents. It has many medicinal uses. Further, it has anti-cancer properties.
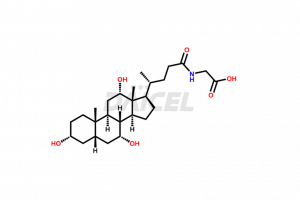
Glycocholic acid Structure and Mechanism of Action
The chemical name of Glycocholic acid is N-[(3α,5β,7α,12α)-3,7,12-Trihydroxy-24-oxocholan-24-yl]glycine. The chemical formula for Glycocholic acid is C26H43NO6, and its molecular weight is approximately 465.62 g/mol.
The precise mechanism of action of Glycocholic acid and its anion, glycocholate, is unclear.
Glycocholic acid Impurities and Synthesis
During Glycocholic acid synthesis, impurities form that may affect the safety and efficacy of the drug. They form during the synthetic process, purification, or storage of Glycocholic acid. Hence, Glycocholic acid impurities need continuous control and monitoring throughout the drug development.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Glycocholic acid impurities, which includes Glycine 6-ethylchenodeoxy cholate Impurity, Glycoursodeoxy cholic acid, Glycochenodeoxy cholic acid, and Ursochenodiol Impurity. We provide the CoA from a cGMP-compliant analytical facility. It gives complete characterization data1,2, like 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We provide additional analytical data on request. Daicel Pharma can prepare any unidentified Glycocholic acid impurity or degradation product. In addition, Daicel Pharma offers highly purified, stable isotope-labeled standards of Glycocholic acid. The clients of Daicel Pharma can expect a complete characterization report on delivery.
References
FAQ's
References
- Nystrom, Ernst; Sjovall, Jan, Thin-layer chromatography of bile acids on lipophilic Sephadex, Acta Chemica Scandinavica (1947-1973), Volume: 21, Issue: 7, Pages: 1974-6, 1967 DOI: (3891/acta.chem.scand.21-1974)
- Panveliwalla, D.; Lewis, B.; Wootton, I. D. P.; Tabaqchali, Soad, Determination of individual bile acids in biological fluids by thin-layer chromatography and fluorimetry, Journal of Clinical Pathology, Volume: 23, Issue: 4, Pages: 309-14, 1970 DOI: (10.1136/jcp.23.4.309)
Frequently Asked Questions
2.Why is it necessary to remove Glycocholic acid impurities from the drug?
Glycocholic acid impurities need removal from the drug to improve quality control, safety, and efficacy while following regulatory guidelines.
3.How do Glycocholic acid degradation products form in the drug?
Glycocholic acid degradation products form during storage under stress conditions like light, moisture, oxidation, etc.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.