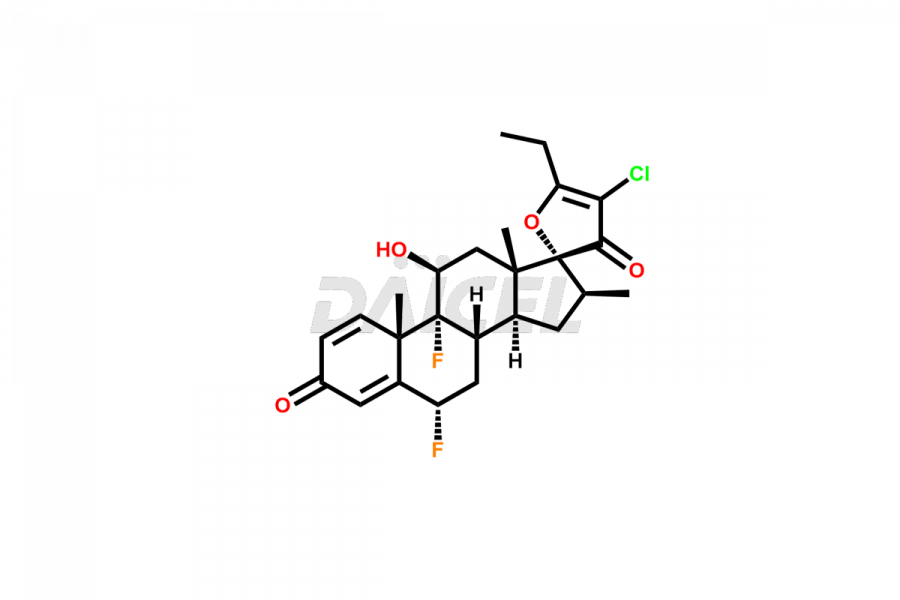
Halobetasol
General Information
Halobetasol Impurities and Halobetasol
Daicel Pharma offers superior-quality Halobetasol impurities, such as Halobetasol Spiro Analog. These impurities are essential for evaluating Halobetasol quality, stability, and biological safety. In addition, Daicel Pharma specializes in the custom synthesis of Halobetasol impurities and ensures their worldwide delivery.
Halobetasol [CAS: 98651-66-2] is a corticosteroid that has anti-inflammatory and immunosuppressive properties. It treats patients with severe or resistant psoriasis. Further, it relieves symptoms due to skin infections.
Halobetasol: Use and Commercial Availability
Halobetasol treats plaque psoriasis and other dermatological conditions. Further, it treats alopecia areata, granuloma annularae, eczema, etc. It provides relief in patients suffering from corticosteroid-responsive dermatoses. Halobetasol prevents and treats symptoms of skin diseases like rash due to Urticaria, dermatitis, itchy skin, and skin redness due to allergies. Manufacturers market it as topical formulations in various dosage forms. Halobetasol is available under brands such as Bryhali, Lexette, Ultravate, etc.
Halobetasol Structure and Mechanism of Action
The chemical name of Halobetasol is (6α,11β,16β)-21-Chloro-6,9-difluoro-11,17-dihydroxy-16-methylpregna-1,4-diene-3,20-dione. The chemical formula for Halobetasol is C22H27ClF2O4, and its molecular weight is approximately 428.90 g/mol.
The precise mechanism of action of Halobetasol is unclear.
Halobetasol Impurities and Synthesis
While synthesizing Halobetasol, impurities may form that will affect drug safety and efficacy. They form during the synthetic process, storage, or purification of Halobetasol. Manufacturers can control and monitor Halobetasol impurities to improve the drug’s safety, efficacy, and storage.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Halobetasol impurities, which includes Halobetasol Spiro Analog. The CoA provided to clients is from a cGMP-compliant analytical facility with the complete characterization data,1,2 such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We give additional spectral data on request. Daicel Pharma can prepare any unidentified Halobetasol impurity or degradation product. In addition, Daicel Pharma offers highly purified, stable isotope-labeled standards of Halobetasol. Daicel Pharma provides a complete characterization report accompanying the delivery.
References
- Prakash, Lakkireddy; Malipeddi, H.; Subbaiah, Venkata B.; Lakka, Narasimha S., Impurity profiling and a stability-indicating UPLC method development and validation for the estimation of related impurities of halobetasol propionate in halobetasol propionate 0.05% (w/w) cream, Journal of Chromatographic Science, Volume: 53, Issue: 1, Pages: 112-121, 2015 DOI: (1093/chromsci/bmu027)
- Nalwade, Santaji; Reddy, Vangala Ranga; Kulkarni, Dipak; Todamal, Sandip, Quantification of halobetasol propionate and its impurities present in topical dosage forms by stability-indicating LC method, Journal of Chromatographic Science, Volume: 53, Issue: 1, Pages: 127-134, 2015 DOI: (10.1093/chromsci/bmu029)
Frequently Asked Questions
2.What are the Halobetasol degradation products found in the drug?
Diflorasone 17 propionate and diflorasone 21 propionate are the Halobetasol degradation products.
3.Under which conditions do Halobetasol degradation products form in the drug?
Alkaline conditions cause the degradation of Halobetasol in the drug.
4.Which analytical method analyzes Halobetasol impurities and degradation products in the drug?
RP-HPLC method helps analyze Halobetasol impurities and degradation products in the drug.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.