LOAD MORE
You're viewed 9 of 12 products
Daicel Pharma offers worldwide delivery options for a custom synthesis of Hydroxychloroquine impurities, including impurities such as Bis-Desethyl chloroquine, Chloroquine N-Oxide, Des ethyl chloroquine, Desethyl Hydroxychloroquine (DHCQ), more. These impurities help evaluate the purity and safety of an active pharmaceutical ingredient, Hydroxychloroquine.
Hydroxychloroquine [CAS: 118-42-3], an aminoquinoline compound, is a variant of chloroquine. It treats lupus erythematosus, rheumatoid arthritis, and light-sensitive skin eruptions, exhibiting properties similar to chloroquine as an antimalarial drug, targeting erythrocytic forms of malarial parasites. Alongside its antimalarial activity, Hydroxychloroquine acts as an antirheumatic agent. It possesses immunosuppressive properties and demonstrates anti-autophagy.
Hydroxychloroquine targets the erythrocytic forms of malarial parasites. Its selective toxicity against the erythrocytic stages of plasmodial infection may be due to its concentration in parasitized erythrocytes. Moreover, as an antirheumatic agent, Hydroxychloroquine suppresses the immune system, causing a decrease in rheumatoid factor and acute phase reactants. It also exhibits an affinity for white blood cells, stabilizing lysosomal membranes and inhibiting enzymes involved in cartilage breakdown, including collagenase and proteases. Notably, Plaquenil is the brand name associated with formulations of this drug.
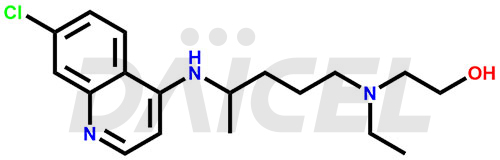
The chemical name of Hydroxychloroquine is 2-[[4-[(7-Chloro-4-quinolinyl)amino]pentyl]ethylamino]ethanol. Its chemical formula is C18H26ClN3O, and its molecular weight is approximately 335.9 g/mol.
Hydroxychloroquine’s mechanism of action is unknown.
Hydroxychloroquine impurities can arise during the manufacturing process, storage, or degradation. The impurities may result from reaction side-products, incomplete reactions, or interactions with other substances. Analytical methods such as HPLC, LC-MS, NMR, and IR spectroscopy help analyze and quantify these impurities. Control measures include optimizing the synthetic process and purification steps and ensuring proper storage conditions to minimize impurity formation. Stability studies assess the impurity profile over time and establish appropriate expiration dates and storage recommendations. Compliance with regulatory guidelines, such as those provided by ICH, is essential to ensure the quality and safety of Hydroxychloroquine formulations. Effective control of impurities plays a vital role in maintaining the integrity of Hydroxychloroquine as a pharmaceutical product.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for synthesizing Hydroxychloroquine impurity standards such as Bis-Desethyl chloroquine, Chloroquine N-Oxide, Des ethyl chloroquine, Desethyl Hydroxychloroquine (DHCQ), and more. We offer deuterium-labeled Hydroxychloroquine standards, including Hydroxychloroquine-D5, essential for bioanalytical research and BA/BE studies. Our impurities have a detailed Certificate of Analysis (CoA) that provides a comprehensive characterization report. This report includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis1,2. Upon request, we give additional data like 13C-DEPT. We can synthesize unknown Hydroxychloroquine impurities or degradation products and labeled compounds. Each delivery has a comprehensive characterization report.
Pharmacopeial standards, such as the United States Pharmacopeia (USP) or the European Pharmacopoeia (EP), outline specific impurity testing requirements for Hydroxychloroquine to ensure its quality and safety.
Impurities in Hydroxychloroquine can influence its stability under specific storage conditions, such as temperature and humidity. Proper storage guidelines help maintain drug integrity.
When analyzing many impurities in Hydroxychloroquine, Methanol is the common solvent.
The recommendation is to store Hydroxychloroquine impurities at a controlled room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.