Iodixanol
General Information
Iodixanol Impurities and Iodixanol
Daicel Pharma offers excellent-quality Iodixanol impurities, such as Iodixanol Impurity-1 (Mixture of Isomers). They are vital for evaluating Iodixanol quality, stability, and biological safety. Furthermore, Daicel Pharma specializes in the custom synthesis of Iodixanol impurities and ensures their worldwide delivery.
Iodixanol [CAS: 92339-11-2] is an iodinated x-ray contrast agent. It is a dimeric, hydrophilic compound that is useful for diagnostic imaging. It helps to view blood vessels and organs on a CT scan. Further, it aids in diagnosing disorders of the brain, heart, kidneys, etc.
Iodixanol: Use and Commercial Availability
During coronary angiography, Iodixanol acts as a contrast agent. As it is less toxic to kidneys, it helps patients with renal dysfunction. It is for CECT imaging of the head and body. Iodixanol visualizes the aorta and its branches to diagnose arterial occlusive diseases, tumors, and aneurysms. The route of drug administration is intravenous or intra-arterial. Iodixanol is available under brands Visipaque 270 and Visipaque 320 as injectable formulations.
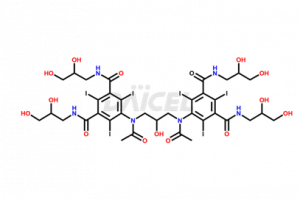
Iodixanol Structure and Mechanism of Action
The chemical name of Iodixanol is 5,5′-[(2-Hydroxy-1,3-propanediyl)bis(acetylimino)]bis[N, N′-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide. The chemical formula for Iodixanol is C35H44I6N6O15, and its molecular weight is approximately 1550.18 g/mol.
Iodixanol opacifies or makes opaque the vessels that are in the path of the flow of the contrast agent. It allows radiographic visualization of the internal organs until noteworthy dilution and elimination occurs.
Iodixanol Impurities and Synthesis
Impurities form during Iodixanol synthesis1 that may affect the safety and efficacy of the drug. These impurities develop during the manufacturing process, purification, or storage of Iodixanol. Therefore, the control and monitoring of Iodixanol impurities is essential throughout the drug development.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Iodixanol impurities, which includes Iodixanol Impurity-1 (Mixture of Isomers). We provide the CoA from a cGMP-compliant analytical facility. It gives complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We provide additional analytical data on request. Daicel Pharma can prepare any unidentified Iodixanol impurity or degradation product. In addition, Daicel Pharma offers highly purified, stable isotope-labeled standards of Iodixanol. We guarantee a complete characterization report on delivery.
References
FAQ's
References
- Hansen, Per Egil; Holtermann, Hugo; Wille, Knut, X-RAY contrast agents, EP108638B1, Jul 16, 1986, Nyegaard og Co. A/S, Norway (https://www.lens.org/lens/search/patent/list?q=EP108638)
- Nomura, Hisashi; Teshima, Emiko; Hakusui, Hideo, Simple isocratic high-performance liquid chromatographic method for measurement of iodixanol in human plasma, Journal of Chromatography, Biomedical Applications, Volume: 572, Issue: 1-2, Pages: 333-8, 1991 DOI: (10.1016/0378-4347(91)80500-c)
Frequently Asked Questions
2.What is the analytical method to identify Iodixanol impurities?
An isocratic hydrophilic interaction liquid chromatographic method helps identify Iodixanol impurities.
3.Why is it crucial to remove the Iodixanol impurities from the drug?
Iodixanol impurities can affect drug safety, quality, and efficacy and need removal from the drug.
4.What causes the degradation of the Iodixanol drug?
Iodixanol degrades on exposure to light, heat, moisture, and improper storage conditions.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.