Lacosamide
General Information
Lacosamide Impurities and Lacosamide
Daicel Pharma offers superior-quality Lacosamide impurities, such as N-Methyl rac-Lacosamide. These impurities are essential for evaluating Lacosamide quality, stability, and biological safety. In addition, Daicel Pharma specializes in the custom synthesis of Lacosamide impurities and ensures their worldwide delivery.
As an amino acid derivative, Lacosamide [CAS: 175481-36-4] treats adult epilepsy patients. It blocks neuronal voltage-gated sodium channels responsible for neuropathic pain responses. It stabilizes hyperexcitable neuronal membranes and blocks repetitive neuronal firing. Lacosamide binds to a phosphoprotein, collapsin response mediator protein-2 (CRMP-2), expressed in the nervous system. However, CRMP-2 role in seizure control is unknown.
Lacosamide: Use and Commercial Availability
Lacosamide is a drug to prevent seizures and is an anticonvulsant. It treats partial-onset seizures in epileptic patients as a supplemental therapy. Further, it helps manage Bilateral Tonic-Clonic Seizure, Focal Aware Onset Seizure, etc. Vimpat and Motpoly XR are the brands under which Lacosamide is marketed as an oral formulation. It is available in various formulations in many countries.
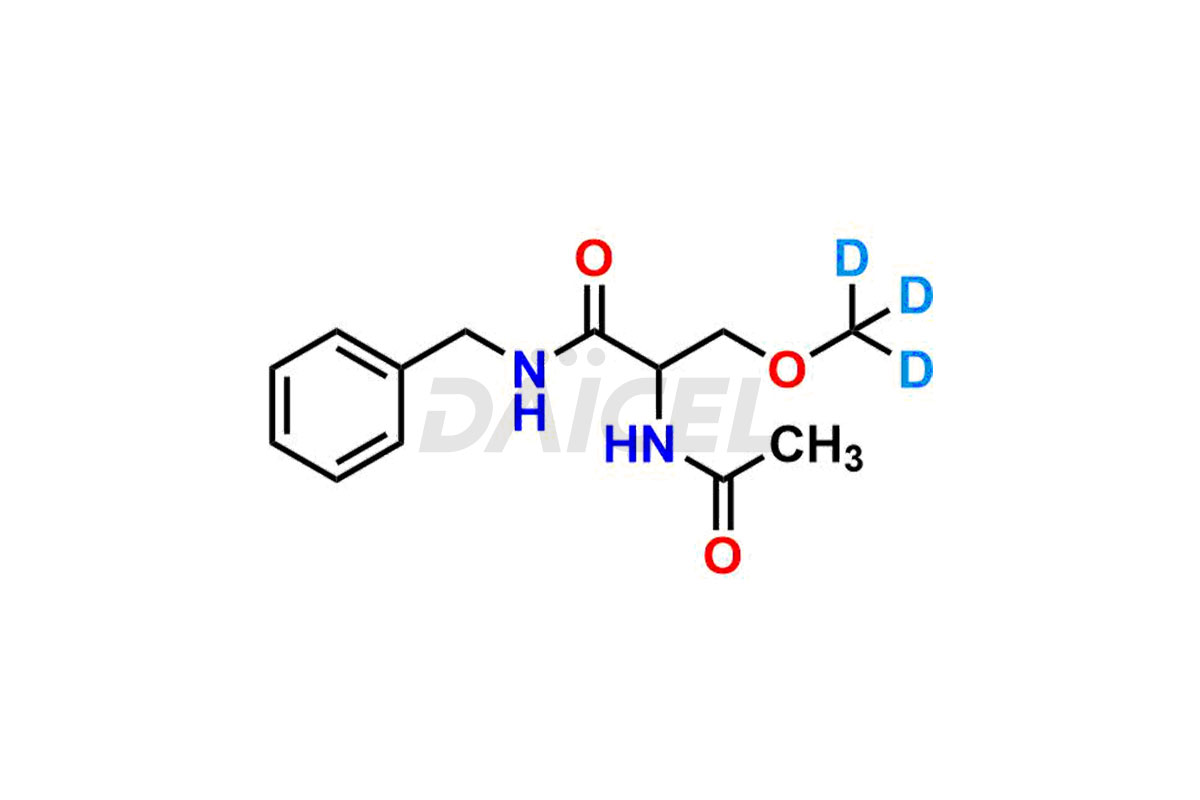
Lacosamide Structure and Mechanism of Action
The chemical name of Lacosamide is (2R)-2-(Acetylamino)-3-methoxy-N-(phenylmethyl)propanamide. The chemical formula for Lacosamide is C13H18N2O3, and its molecular weight is approximately 250.29 g/mol.
The precise mechanism of action of Lacosamide is unclear.
Lacosamide Impurities and Synthesis
While synthesizing Lacosamide 1, impurities may form that will affect drug safety and efficacy. These impurities develop during the Lacosamide synthesis, storage, or purification process. Manufacturers can control and monitor Lacosamide impurities to improve the drug’s safety, efficacy, and storage.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Lacosamide impurities, which includes N-Methyl rac-Lacosamide. The CoA provided to clients is from a cGMP-compliant analytical facility, with complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional spectral data on request. Daicel Pharma can prepare any unidentified Lacosamide impurity or degradation product. Further, Daicel Pharma offers a highly purified, stable deuterium-labeled standard, Lacosamide-D3. We provide clients with a complete characterization report upon delivery.
References
FAQ's
References
- Kohn, Harold, Anticonvulsant enantiomeric amino acid derivatives, WO9733861A1, Sep 18, 1997, Research Corporation Technologies, Inc., United States (https://patents.google.com/patent/WO1997033861A1/en)
- Kestelyn, C.; Lastelle, M.; Higuet, N.; Dell''Aiera, S.; Staelens, L.; Boulanger, P.; Boekens, H.; Smith, S., A simple HPLC-UV method for the determination of lacosamide in human plasma, Bioanalysis, Volume: 3, Issue: 22, Pages: 2515-2522, 2011 DOI: (10.4155/bio.11.261)
Frequently Asked Questions
2.What causes the formation of Lacosamide impurities?
Incomplete reaction conditions and reagents during the synthetic process are the source of Lacosamide impurities.
3.How does the Lacosamide drug degrade?
Lacosamide degrades under oxidative, alkaline, and acid hydrolytic conditions.
4.Which analytical technique identify Lacosamide degradation products?
Liquid chromatography hybrid ion trap/time-of-flight mass spectrometry (LC-IT/TOF-MS) and Liquid chromatography hybrid triple quadrupole-linear ion trap mass spectrometry (LC–QqLIT-MS) identify Lacosamide degradation products.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.