LOAD MORE
You're viewed 9 of 10 products
Daicel Pharma synthesizes high-quality Lamivudine impurities like 2′-Epi Lamivudine, Lamivudine Enantiomer, Lamivudine impurity G, Lamivudine impurity H, Lamivudine impurity J, and Trans-Lamivudine, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Lamivudine. Moreover, Daicel Pharma offers custom synthesis of Lamivudine impurities and delivers them globally.
Lamivudine [CAS: 134678-17-4] is a synthetic nucleoside analog that functions as a reverse transcriptase inhibitor and is employed to treat individuals with human immunodeficiency virus (HIV) and hepatitis B virus (HBV) infections.
Lamivudine is a prescription medicine that belongs to the nucleoside reverse transcriptase inhibitor (NRTI) class, which is used in combination with other drugs to treat human immunodeficiency virus type-1 (HIV-1) and as a monotherapy for hepatitis B virus (HBV) infections. It reduces the viral load but does not eradicate the virus from the human body. Lamivudine is available in tablet form and as an oral solution and is also included in several co-formulations such as Cimduo, Dovato, Triumeq. Epivir is the trade name for Lamivudine.
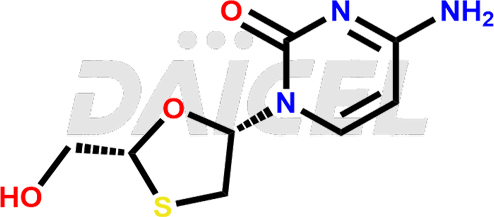
The chemical name of Lamivudine is 4-Amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-2(1H)-pyrimidinone. Its chemical formula is C8H11N3O3S, and its molecular weight is approximately 229.26 g/mol.
Lamivudine is phosphorylated to lamivudine triphosphate (L-TP), its active 5′-triphosphate metabolite. It inhibits HIV-1 reverse transcriptase (RT) via DNA chain termination after incorporation into viral DNA.
Lamivudine forms impurities during its manufacturing process1, storage, and transportation. Impurities arise from various sources, such as starting materials, solvents, reagents, or degradation products. They can affect the quality, safety, and efficacy of the drug product, and hence, their control is crucial. Strict regulatory guidelines require monitoring and controlling impurities within acceptable limits throughout the drug’s life cycle to ensure the quality of Lamivudine.
Daicel provides a Certificate of Analysis (CoA) for Lamivudine impurity standards, 2′-Epi Lamivudine, Lamivudine Enantiomer, Lamivudine impurity G, Lamivudine impurity H, Lamivudine impurity J, and Trans-Lamivudine. The CoA is issued from a cGMP-compliant analytical facility and contains complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additional characterization data, such as 13C-DEPT and CHN, can be provided upon request. Daicel can also prepare any unknown Lamivudine impurity or degradation product. We give a complete characterization report on delivery.
Impurities in Lamivudine are identified and quantified using various analytical methods, such as high-performance liquid chromatography (HPLC) and Liquid chromatography-mass spectroscopy (LC-MS). These methods separate, identify, and quantify the impurities present in the drug product.
Impurities are controlled in the manufacturing of Lamivudine by using appropriate synthesis, purification, and formulation processes. Also, proper storage conditions and packaging prevents the formation and accumulation of impurities.
The presence of impurities in Lamivudine can affect the drug's efficacy, as they can interfere with the drug's mechanism of action or alter its pharmacokinetic profile.
Lamivudine impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.