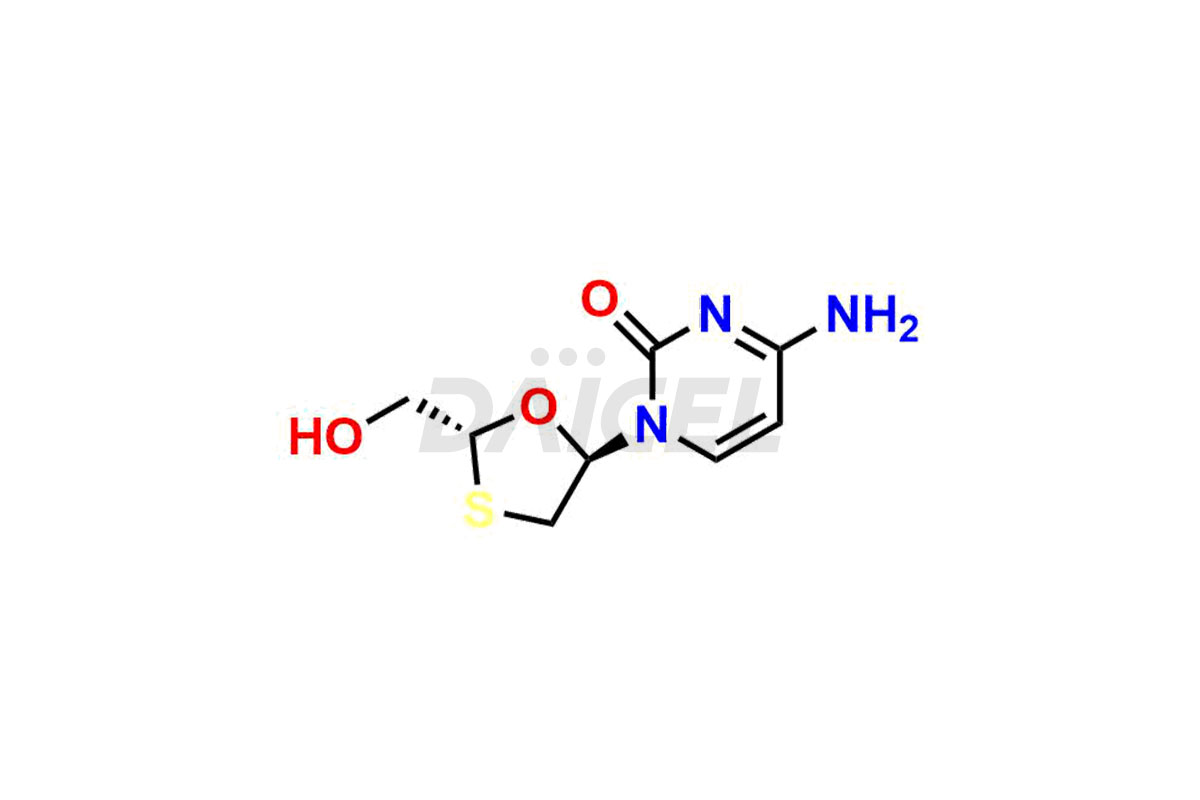
Lamivudine
References
- Coates, Jonathan Alan Victor; Mutton, Ian Martin; Penn, Charles Richard; Storer, Richard; Williamson, Christopher, 1,3-oxathiolane nucleoside analogues, IAF Biochem International Inc., Canada, EP625150A1 1, November 14, 1991
- Hsyu, Poe-Hirr; Lloyd, Thomas L., Automated high-performance liquid chromatographic analysis of (-)-2'-deoxy-3'-thiacytidine in biological fluids using the automated sequential trace enrichment of dialyzate systems, Journal of Chromatography B: Biomedical Sciences and Applications, Volume: 655, Issue: 2, Pages: 253-60, 1994
Frequently Asked Questions
How are Lamivudine impurities identified and quantified?
Impurities in Lamivudine are identified and quantified using various analytical methods, such as high-performance liquid chromatography (HPLC) and Liquid chromatography-mass spectroscopy (LC-MS). These methods separate, identify, and quantify the impurities present in the drug product.
How are the impurities controlled in the manufacturing of Lamivudine?
Impurities are controlled in the manufacturing of Lamivudine by using appropriate synthesis, purification, and formulation processes. Also, proper storage conditions and packaging prevents the formation and accumulation of impurities.
Can the presence of Lamivudine impurities affect the efficacy of the drug?
The presence of impurities in Lamivudine can affect the drug's efficacy, as they can interfere with the drug's mechanism of action or alter its pharmacokinetic profile.
What are the temperature conditions required to store Lamivudine impurities?
Lamivudine impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.