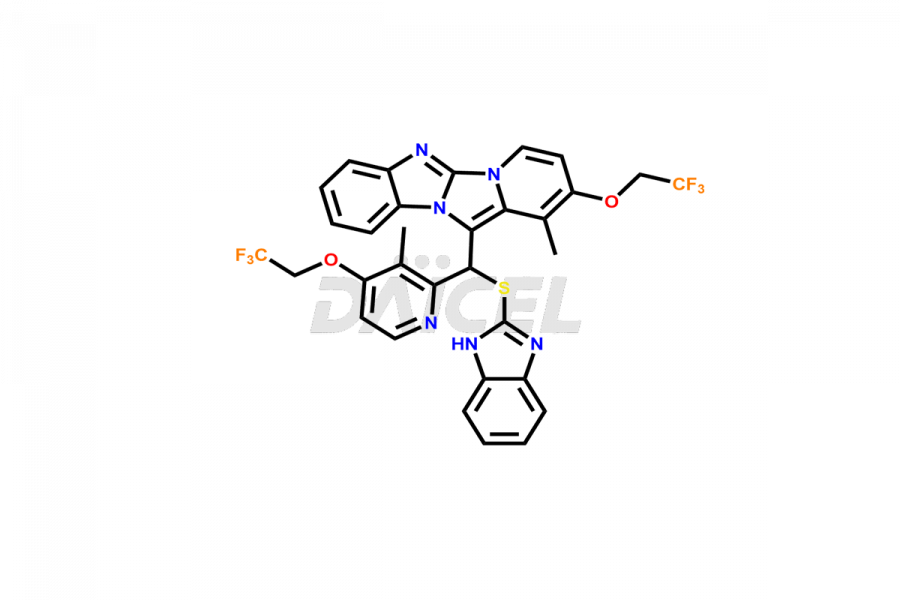
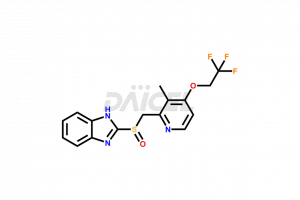
Lansoprazole
General Information
Lansoprazole Impurities and Lansoprazole
Daicel Pharma offers superior-quality Lansoprazole impurities, such as Dexlansoprazole Impurity-II. These impurities are essential for evaluating Lansoprazole quality, stability, and biological safety. In addition, Daicel Pharma specializes in the custom synthesis of Lansoprazole impurities and ensures their worldwide delivery.
Lansoprazole [CAS: 103577-45-3] is a benzimidazole derivative. It is a proton pump inhibitor (PPI) that reduces acid secretion in the stomach. It is an anti-ulcer agent that treats or prevents peptic ulcers. It prevents gastric acid secretion by irreversibly blocking the proton pump system.
Lansoprazole: Use and Commercial Availability
Lansoprazole treats gastric and peptic ulcers as it is a gastric acid pump inhibitor. It also treats reflux esophagitis, indigestion, and heartburn. It treats symptoms of gastroesophageal reflux disease (GERD) in patients. Further, it manages Zollinger-Ellison syndrome, the cause of a tumor in the pancreas or gut. It helps treat gastric ulcers caused by chronic use of non-steroidal anti-inflammatory drugs (NSAID) by patients. Lansoprazole is available as an oral formation under the brand Prevacid.
Lansoprazole Structure and Mechanism of Action
The chemical name of Lansoprazole is 2-[[[3-Methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methyl]sulfinyl]-1H-benzimidazole. The chemical formula for Lansoprazole is C16H14F3N3O2S, and its molecular weight is approximately 369.36 g/mol.
Lansoprazole blocks the (H+, K+)-ATPase enzyme system at the secretory surface of the gastric parietal cells. It thus leads to the prevention of gastric acid production in the final step.
Lansoprazole Impurities and Synthesis
While synthesizing Lansoprazole 1, impurities may form that will affect drug safety and efficacy. They form during the synthetic process, storage, or purification of Lansoprazole. Manufacturers can control and monitor Lansoprazole impurities to improve the drug’s safety, efficacy, and storage.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Lansoprazole impurities, which includes Dexlansoprazole Impurity-II. The CoA provided to clients is from a cGMP-compliant analytical facility, with complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional spectral data on request. Daicel Pharma can prepare any unidentified Lansoprazole impurity or degradation product. In addition, Daicel Pharma offers highly purified, stable isotope-labeled standards of Lansoprazole. We also provide a complete characterization report along with the delivery.
References
- Nohara, Akira; Maki, Yoshitaka, Pyridine derivatives and their production, EP174726B1, Apr 26, 1989, Takeda Chemical Industries, Ltd., Japan (https://www.lens.org/lens/search/patent/list?q=EP0174726)
- Aoki, Isamu; Okumura, Minoru; Yashiki, Takatsuka, High-performance liquid chromatographic determination of lansoprazole and its metabolites in human serum and urine, Journal of Chromatography, Biomedical Applications, Volume: 571, Issue: 1-2, Pages: 283-90, 1991 DOI: (10.1016/0378-4347(91)80457-n)
Frequently Asked Questions
2.What causes the degradation of the Lansoprazole drug?
Lansoprazole degrades under acid and alkaline hydrolysis.
3.What are the different Lansoprazole degradation products identified in the drug?
7-(3-Methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)-7H-benzo[4,5]imidazo[2,1-b]benzo[4,5]imidazo [2,1-d]- [1,3,5]thiadiazine and 1-Methyl-10-thioxo-10H-4a,5,9b-triaza-indeno[2,1-a]inden-2-one are the different Lansoprazole degradation products identified in the drug.
4.How are unknown Lansoprazole degradation products identified in the drug?
LC-MS helps to identify the unknown Lansoprazole degradation products in the drug.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.