Ledipasvir
General Information
Ledipasvir Impurities and Ledipasvir
Daicel Pharma offers excellent-quality Ledipasvir impurities and labeled standards. They are vital for evaluating Ledipasvir quality, stability, and biological safety. Furthermore, Daicel Pharma specializes in the custom synthesis of Ledipasvir impurities and ensures their worldwide delivery.
Ledipasvir [CAS: 1256388-51-8] is an antiviral drug and a benzimidazole derivative. It inhibits the Hepatitis C Virus NS5A protein, essential for viral RNA replication. As a direct-acting antiviral, Ledipasvir interferes with HCV replication. Developed by Gilead Sciences, it has antiviral activity against the Hepatitis C Virus (HCV).
Ledipasvir: Use and Commercial Availability
Ledipasvir, in combination with Sofosbuvir, treats chronic hepatitis C Virus infection under the brand Harvoni. It treats HCV genotypes 1, 4,5, or 6 infections in patients without cirrhosis. It is available under various brands in combination with other drugs such as Ribavirin, Sofosbuvir, and other antivirals.
Ledipasvir Structure and Mechanism of Action
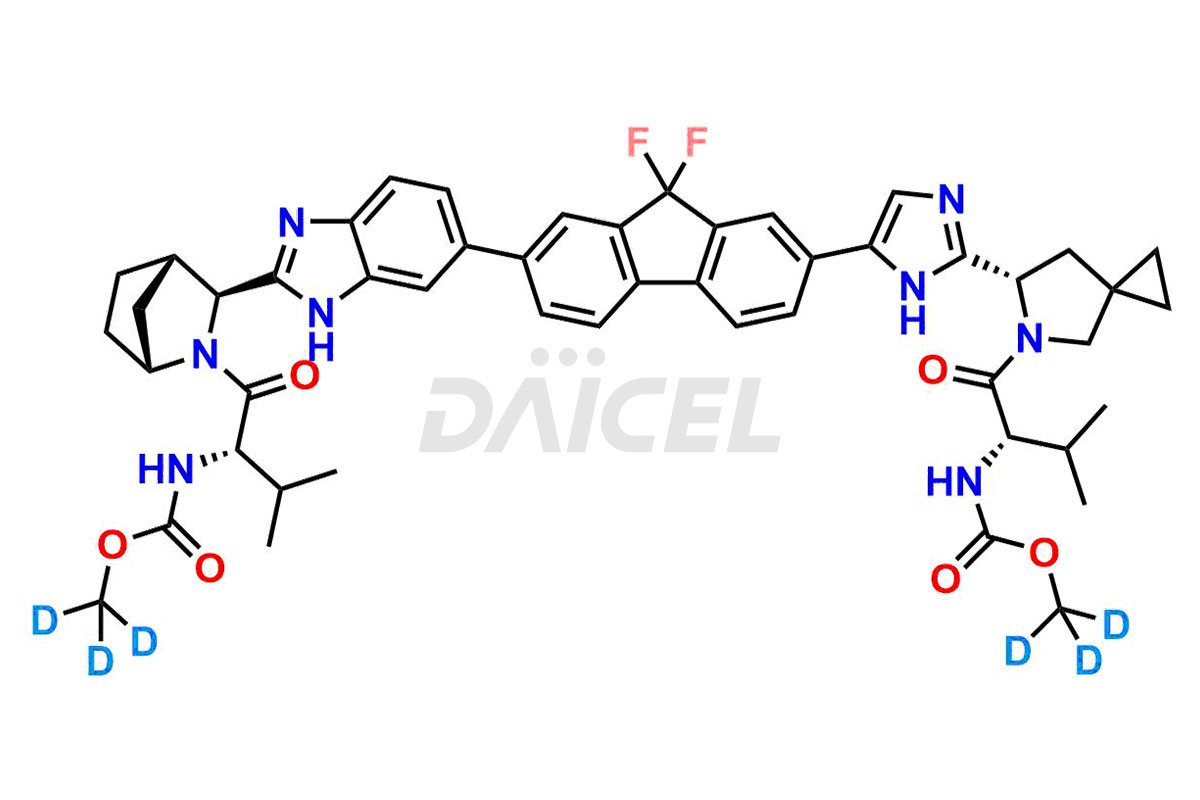
The chemical name of Ledipasvir is N-[(1S)-1-[[(6S)-6-[5-[9,9-difluoro-7-[2-[(1R,3S,4S)-2-[(2S)-2-[(methoxycarbonyl)amino]-3-methyl-1-oxobutyl]-2-azabicyclo[2.2.1]hept-3-yl]-1H-benzimidazol-6-yl]-9H-fluoren-2-yl]-1H-imidazol-2-yl]-5-azaspiro[2.4]hept-5-yl]carbonyl]-2-methylpropyl]-Carbamic acid methyl ester. The chemical formula for Ledipasvir is C49H54F2N8O6, and its molecular weight is approximately 889.00 g/mol.
The precise mechanism of action of Ledipasvir is unknown.
Ledipasvir Impurities and Synthesis
Impurities form during Ledipasvir that may affect the drug’s safety and efficacy. These impurities develop during the manufacturing process, purification, or storage of Ledipasvir. Therefore, the control and monitoring of Ledipasvir impurities is essential throughout the drug development.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Ledipasvir impurities and labeled standards. We provide the CoA from a cGMP-compliant analytical facility. It gives complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We provide additional analytical data on request. Daicel Pharma can prepare any unidentified Ledipasvir impurity or degradation product. In addition, Daicel Pharma offers a highly purified, stable deuterium-labeled standard, Ledipasvir Labelled Standard. We guarantee a complete characterization report on delivery.
References
- Guo, Hongyan; Kato, Darryl; Kirschberg, Thorsten A.; Liu, Hongtao; Link, John O.; Mitchell, Michael L.; Parrish, Jay P.; Squires, Neil; Sun, Jianyu; Taylor, James; et al, Antiviral compounds, WO2010132601A1, Nov 18, 2010, Gilead Sciences, Inc., United States (https://patents.google.com/patent/WO2010132601A1/en)
- Devilal, J.; Durgaprasad, B.; Pal, Narottam; Rao, A. Srinivasa, New method development and validation for the determination of Ledipasvir in bulk drug form by using reverse phase HPLC technique, World Journal of Pharmacy and Pharmaceutical Sciences, Volume: 5, Issue: 8, Pages: 1312-1321, 2016 DOI: (10.20959/wjpps20168-7410)
Frequently Asked Questions
2.What is the analytical method to identify Ledipasvir impurities?
A reverse-phase high-performance liquid chromatography method helps identify Ledipasvir impurities.
3.What causes the degradation of the Ledipasvir drug?
Ledipasvir degrades under oxidative, alkaline, and acid conditions.
4.Which analytical technique identifies the Ledipasvir degradation products in the drug?
Ledipasvir degradation products are identified using the LC-MS/MS analytical method.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.