LOAD MORE
You're viewed 9 of 21 products
Daicel Pharma offers worldwide delivery options for custom synthesis of Lenalidomide impurity standards, including crucial impurity standards such as 4-Nitrophthalide (Lenalidomide KSM lactone impurity), Lenalidomide Amadori impurity, Lenalidomide Impurity-3, Lenalidomide open-chain Amadori impurity, Methyl 2-(bromomethyl)-4-nitrobenzoate, Methyl 2-(bromomethyl)-5-nitrobenzoate, Methyl 2-(bromomethyl)-6-nitrobenzoate, and Methyl 2-(chloromethyl)-3-nitrobenzoate. These impurity standards play a vital role in evaluating the purity and safety of Lenalidomide, an active pharmaceutical ingredient.
Lenalidomide [CAS: 191732-72-6] is an analog of thalidomide with anti-cancer properties. It is an immunomodulator and anti-neoplastic agent treating multiple myeloma. Derived from phthalimide and piperidone, Lenalidomide exhibits immunomodulatory and anti-angiogenic characteristics. It manages transfusion-dependent anemia in myelodysplastic syndromes and relapsed or refractory mantle cell lymphoma.
Lenalidomide, available as Revlimid, is a US FDA-approved analog of thalidomide used for treating various conditions. It manages myelodysplastic syndrome (MDS) in patients with transfusion-dependent anemia or genetic abnormality. Lenalidomide is also for refractory or relapsed multiple myeloma, either as monotherapy or in combination with dexamethasone, bortezomib, and dexamethasone, or melphalan and prednisone for newly diagnosed patients who are ineligible for transplant. Additionally, it treats relapsed or refractory mantle cell lymphoma.
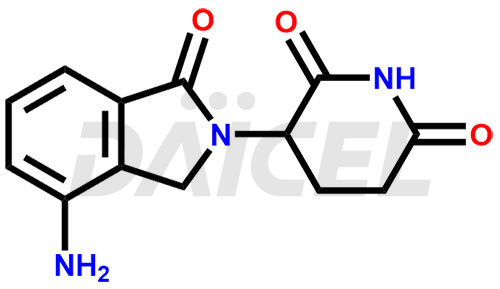
The chemical name of Lenalidomide is 3-(4-Amino-1,3-dihydro-1-oxo-2H-isoindol-2-yl)-2,6-piperidinedione. Its chemical formula is C13H13N3O3, and its molecular weight is approximately 259.26 g/mol.
Lenalidomide prevents the secretion of pro-inflammatory cytokines and raises the secretion of anti-inflammatory cytokines.
During the manufacturing1 of Lenalidomide, impurities can form as byproducts. They can affect the quality and safety of the drug. Various analytical techniques, such as high-performance liquid chromatography (HPLC), helps identify and quantify these impurities. Strict control measures ensure the impurity levels remain within acceptable limits. The impurity profile of Lenalidomide is closely monitored throughout the manufacturing process to maintain its purity and potency.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for preparing Lenalidomide impurity standards. We provide a range of Lenalidomide impurity standards, such as 4-Nitrophthalide (Lenalidomide KSM lactone impurity), Lenalidomide Amadori impurity, Lenalidomide Impurity-3, Lenalidomide open-chain Amadori impurity, Methyl 2-(bromomethyl)-4-nitrobenzoate, Methyl 2-(bromomethyl)-5-nitrobenzoate, Methyl 2-(bromomethyl)-6-nitrobenzoate, and Methyl 2-(chloromethyl)-3-nitrobenzoate. Our impurities have a detailed Certificate of Analysis (CoA) and a comprehensive characterization report. The CoA encompasses data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Additional data, such as 13C-DEPT, can be provided upon request. We can synthesize unknown Lenalidomide impurity standards or degradation products. Each delivery has a comprehensive characterization report.
Analytical techniques such as high-performance liquid chromatography (HPLC), liquid chromatography (LC), or mass spectrometry (MS) help detect and quantify Lenalidomide impurities.
Stringent quality control measures are implemented during the manufacturing process of Lenalidomide to minimize impurities. It includes monitoring and optimizing reaction conditions, purification steps, and storage conditions.
Yes, impurities in Lenalidomide can contribute to the degradation and reduce its stability over time, potentially impacting its shelf life and therapeutic effectiveness.
The recommendation is to store Lenalidomide impurities at a controlled room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.