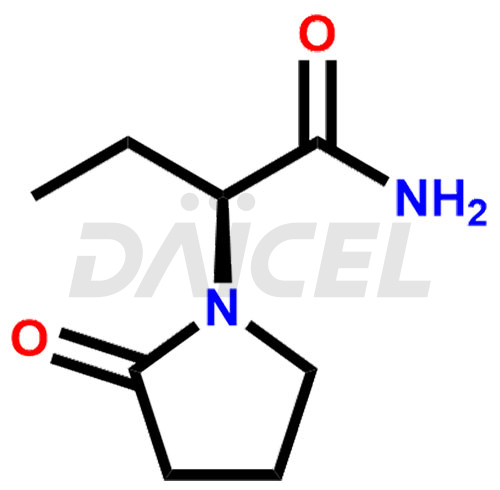
Levetiracetam
General Information
Levetiracetam Impurities and Levetiracetam
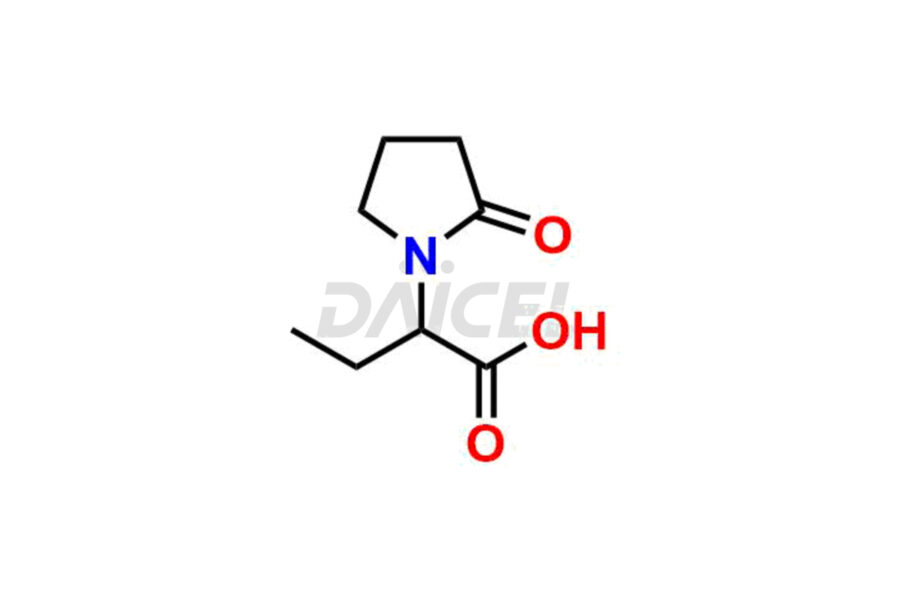
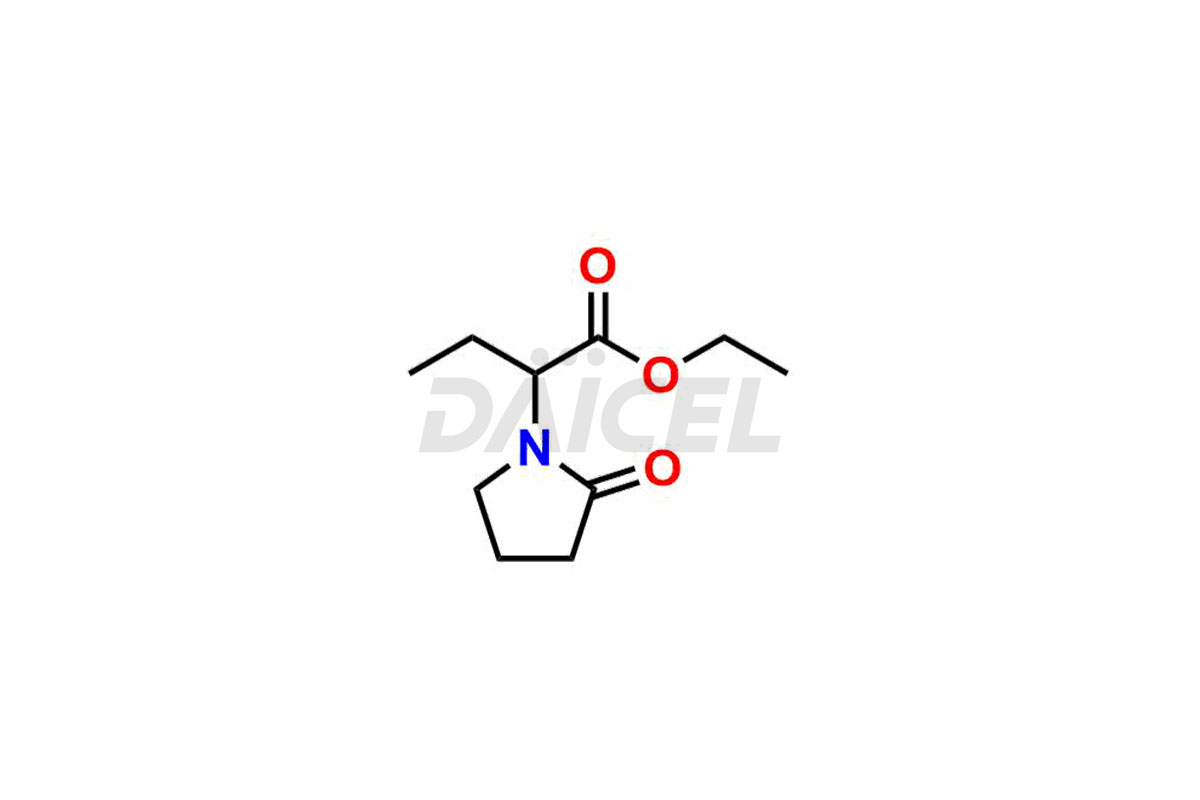
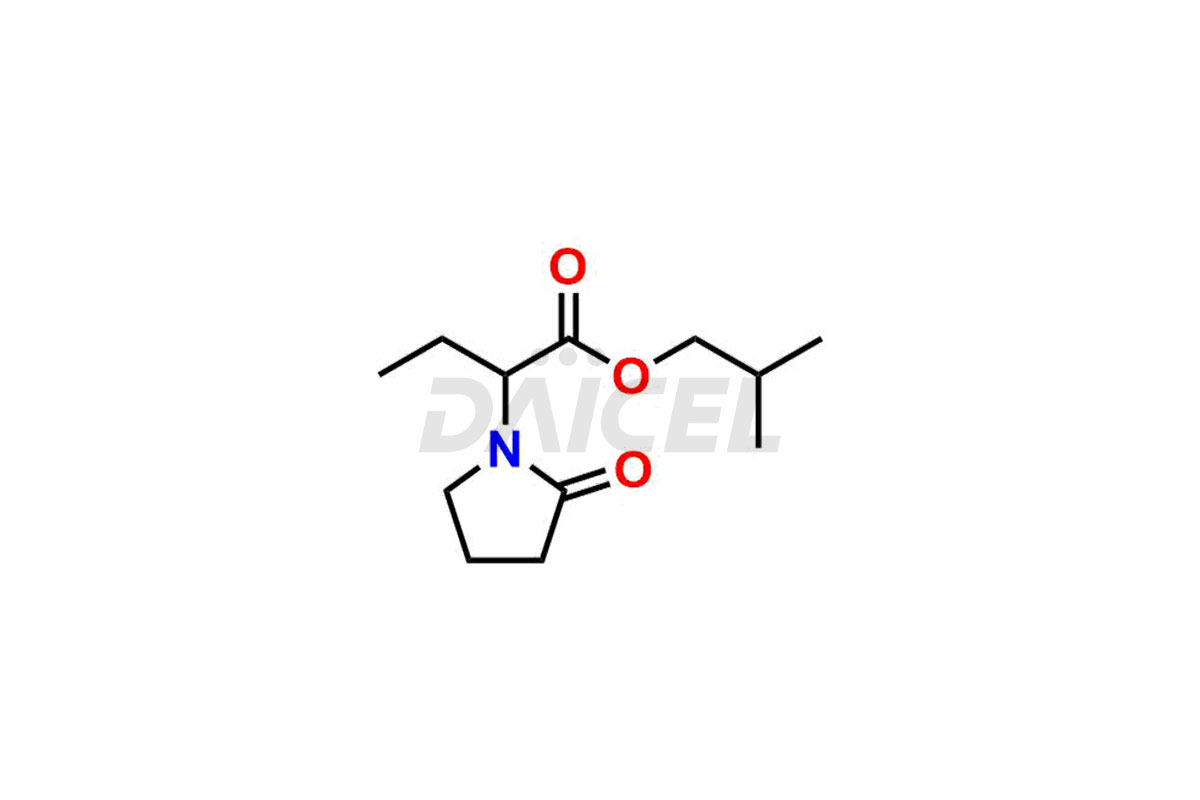
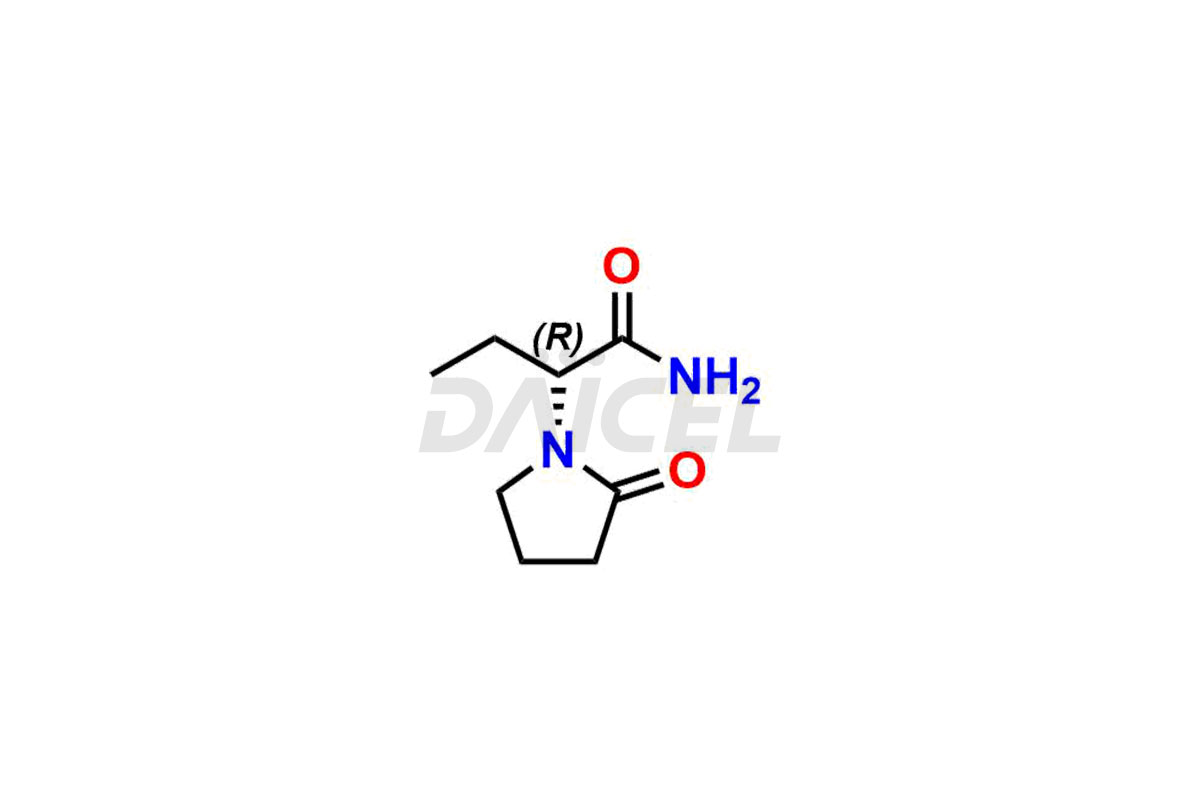
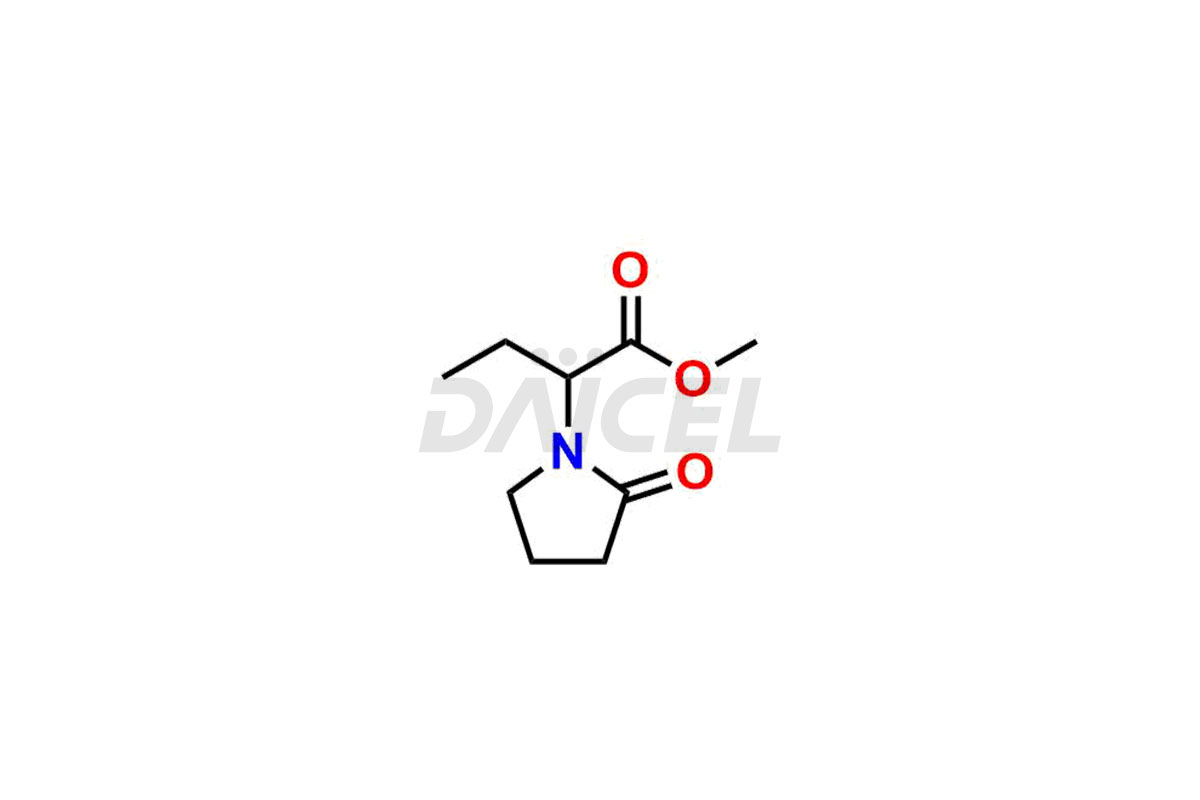
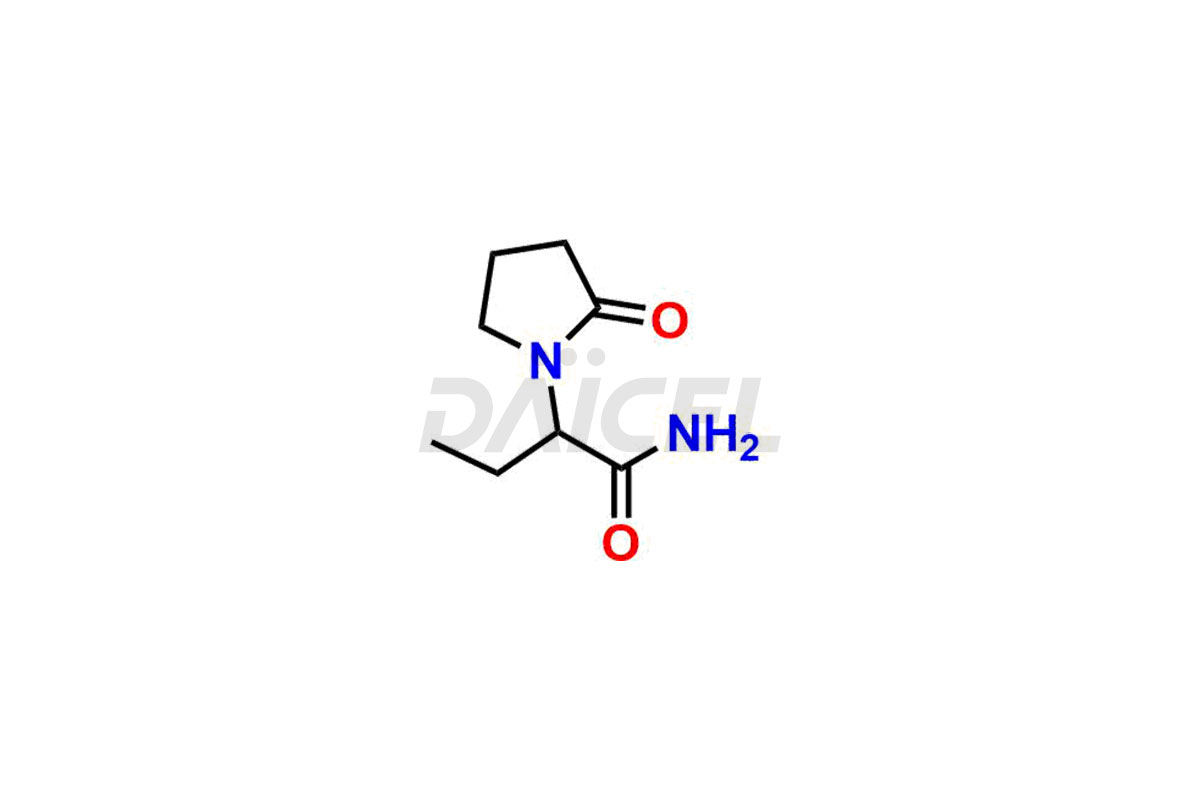
Daicel Pharma offers worldwide delivery options for custom synthesis of Levetiracetam impurity standards, including crucial impurity standards such as 2-(2-Oxopyrrolidin-1-yl)butanoic acid, Ethyl 2-(2-oxopyrrolidin-1-yl)butanoate, Isobutyl 2-(2-oxopyrrolidin-1-yl) butanoate, Levetiracetam Enantiomer, methyl 2-(2-oxopyrrolidin-1-yl) butanoate, and rac-Levetiracetam / Etiracetam. These impurity standards play a vital role in evaluating the purity and safety of Levetiracetam, an active pharmaceutical ingredient.
Levetiracetam [CAS: 102767-28-2] is a pyrrolidine derivative with antiepileptic activity. It combines with other antiepileptic medications for treating partial-onset seizures. Additionally, Levetiracetam treats seizures and movement disorders and is a nootropic agent.
Levetiracetam: Use and Commercial Availability
The FDA-approved Levetiracetam treats focal seizures, myoclonic seizures, and primary generalized seizures as adjunctive therapy. It is available under brand names such as Elepsia XR, Keppra, Keppra XR, and Spritam. Levetiracetam is a novel antiepileptic drug treating partial, myoclonic, and tonic-clonic seizures.
Levetiracetam Structure and Mechanism of Action
The chemical name of Levetiracetam is (αS)-α-Ethyl-2-oxo-1-pyrrolidineacetamide. Its chemical formula is C8H14N2O2, and its molecular weight is approximately 170.21 g/mol.
The mechanism of action of Levetiracetam is unknown.
Levetiracetam Impurities and Synthesis
Impurities in Levetiracetam can generate during the manufacturing process1, either as byproducts or degradation products. They may arise from the starting materials, synthetic steps, or storage conditions. Analytical techniques such as high-performance liquid chromatography (HPLC) help identify and quantify impurities. Strict control measures ensure impurity levels are within acceptable limits, ensuring the quality and safety of Levetiracetam. Thorough analysis and monitoring throughout the manufacturing process help maintain the efficacy and purity of the drug.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for preparing impurity standards. We provide a range of Levetiracetam impurity standards, such as 2-(2-Oxopyrrolidin-1-yl)butanoic acid, Ethyl 2-(2-oxopyrrolidin-1-yl)butanoate, Isobutyl 2-(2-oxopyrrolidin-1-yl) butanoate, Levetiracetam Enantiomer, methyl 2-(2-oxopyrrolidin-1-yl) butanoate, and rac-Levetiracetam / Etiracetam. Our impurity standards have a detailed Certificate of Analysis (CoA) and a comprehensive characterization report. The CoA encompasses data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Additional data, such as 13C-DEPT, can be provided upon request. We can synthesize unknown Levetiracetam impurity standards or degradation products. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Gobert, Jean; Geerts, Jean Pierre; Bodson, Guy, (S)-Alpha-Ethyl-2-Oxo-1-Pyrrolidinacetamide, UCB S. A., Belgium, EP162036B1, August 16, 1989
- Vermeij, T. A. C.; Edelbroek, P. M., High-performance liquid chromatographic and megabore gas-liquid chromatographic determination of levetiracetam (ucb L059) in human serum after solid-phase extraction, Journal of Chromatography B: Biomedical Sciences and Applications, Volume: 662, Issue: 1, Pages: 134-9, 1994
Frequently Asked Questions
Are there any known interactions between Levetiracetam impurities and other drugs or substances?
Interactions between impurities in Levetiracetam and other drugs help minimize potential interactions that could affect drug efficacy or safety.
Are Levetiracetam impurities classified based on their toxicity levels?
Yes, impurities in Levetiracetam can be classified based on their toxicity levels. Regulatory guidelines often provide thresholds and limits for specific classes of impurities to ensure patient safety.
Can Levetiracetam impurities be monitored during clinical trials?
Yes, impurity monitoring is an essential part of clinical trials for Levetiracetam. It helps assess the drug's stability, potential interactions, and safety profile.
How should Levetiracetam impurities be stored in terms of temperature?
The recommendation is to store Levetiracetam impurities at a controlled room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.