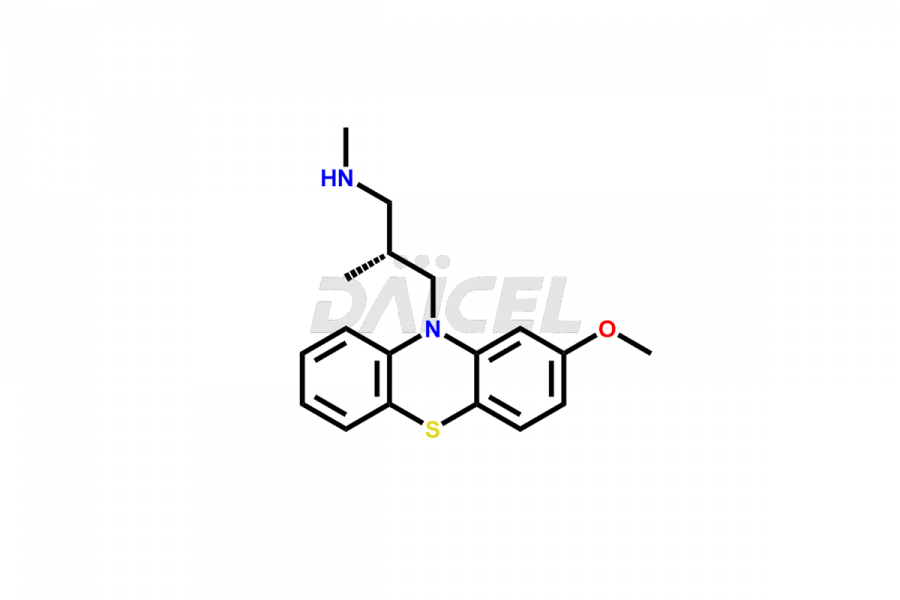
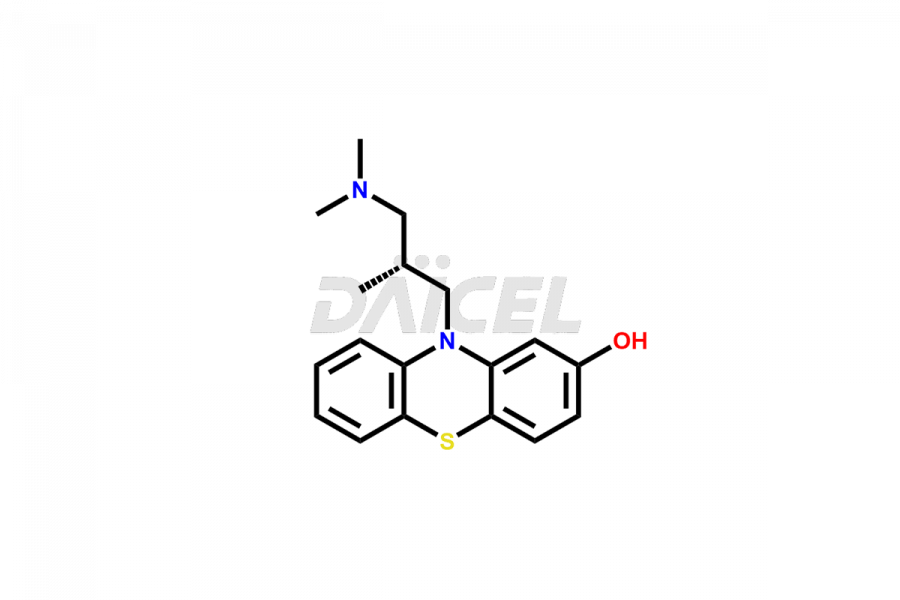
Levomepromazine
General Information
Levomepromazine Impurities and Levomepromazine
Daicel Pharma offers high-quality Levomepromazine impurities, such as N-Desmethyl-Levomepromazine and O-Desmethyl-Levomepromazine. It is vital for evaluating Levomepromazine quality, stability, and biological safety. In addition, Daicel Pharma specializes in the custom synthesis of Levomepromazine impurities and ensures their worldwide delivery.
Levomepromazine [CAS: 60-99-1] is an antipsychotic medicine. It is a phenothiazine derivative and a dopamine antagonist. Further, Levomepromazine is an antiemetic, analgesic and neuroleptic. It has antihistamine and antipsychotic properties.
Levomepromazine: Use and Commercial Availability
Levomepromazine treats psychosis, nausea, and vomiting in patients. It acts as a pre- and post-operative sedative, analgesic, and insomnia management. In addition, it is an antihistamine. Levomepromazine also acts as a tranquilizer that enhances the action of hypnotic medicines. Many generic manufacturers market Levomepromazine under different names. In the US, Levomepromazine is available as a parenteral under the brand Levoprome. In Europe, it is available as Nozinan and Neurocil.
Levomepromazine Structure and Mechanism of Action

The chemical name of Levomepromazine is (2R)-3-(2-Methoxyphenothiazin-10-yl)-N, N,2-trimethylpropan-1-amine. The chemical formula for Levomepromazine is C19H24N2OS, and its molecular weight is approximately 328.47 g/mol.
Levomepromazine blocks dopamine-2-receptor activity. The exact mechanism of action of Levomepromazine is not clearly understood.
Levomepromazine Impurities and Synthesis
While preparing Levomepromazine 1, impurities formation may affect drug safety, efficacy, and shelf-life. They may form during the synthesis, storage, or degradation of Levomepromazine. It is necessary to control and monitor the Levomepromazine impurities to prevent any adverse effects on drug efficacy and safety.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Levomepromazine impurities, which includes N-Desmethyl-Levomepromazine and O-Desmethyl-Levomepromazine. The CoA is from a cGMP-compliant analytical facility. It contains the complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional data like 13C-DEPT and CHN on request. Daicel Pharma can prepare any unidentified Levomepromazine impurity or degradation product. In addition, Daicel Pharma offers highly purified isotope-labeled standards of Levomepromazine for bioanalytical research and BA/BE studies. We also provide a complete characterization report on delivery.
References
FAQ's
References
- Robert Michel Jacob, Ablon-sur-Seine, and Jacques, Georges Robert, Paris, France, Phenthiazine compounds, US2837518A, Nov 8, 1954, Societe des Usines Chimiques Rhone-Ponlenc, Paris, France
- Loennechen, T.; Dahl, S. G., High-performance liquid chromatography of levomepromazine (methotrimeprazine) and its main metabolites, Journal of Chromatography, Volume: 503, Issue: 1, Pages: 205-15, 1990 DOI: (10.1016/s0021-9673(01)81502-8)
Frequently Asked Questions
What are the chief sources of Levomepromazine impurities?
Drug substance degradation and by-products formed during synthesis are the chief sources of Levomepromazine impurities.
Which analytic method characterizes Levomepromazine impurities?
HPLC is the analytical method that characterizes Levomepromazine impurities.
What are the regulatory requirements for studying Levomepromazine impurities?
The regulatory requirements are Method validation, acceptance criteria determination, safety and quality of drugs, evaluating shelf-life, threshold limits, and study of stability and storage conditions.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.