LOAD MORE
You're viewed 9 of 28 products
For the purity and safety of Levothyroxine, an active pharmaceutical ingredient, Daicel Pharma offers a customized synthesis of Levothyroxine impurity standards. These impurity standards include crucial compounds such as 3,3′,5-Triiodothyroacetic acid, 3, 5- Diiodo- L- thyronine, 3,5-diiodo-L-tyrosine, Levothyroxine Impurity F, Levothyroxine Impurity-J, Levothyroxine-N-Lactoside, T4-Aldehyde (Impurity-I), and more. Additionally, Daicel Pharma provides worldwide delivery options for Levothyroxine impurity standards.
Levothyroxine [CAS: 51-48-9] is a synthetic version of thyroxine, which functions as a thyroid hormone and an antithyroid medication. Triiodothyronine (T3) exerts a range of metabolic stimulation on cells. Levothyroxine, chemically resembling the natural thyroid hormone, mimics its functions and counteracts thyroid-related conditions.
Levothyroxine, available under brand names such as Euthyrox, Levo-T, Synthroid, Levolet, Levoxyl, Thyquidity, Thyro-Tabs, Tirosint, Tirosint-Sol, Unithroid, etc., is an oral medication primarily used to treat various forms of hypothyroidism. Additionally, Levothyroxine has received US FDA approval for suppressing pituitary thyrotropin as an adjunct treatment for well-differentiated thyroid cancer dependent on thyrotropin.
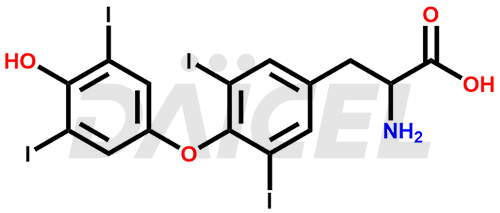
The chemical name of Levothyroxine is O-(4-Hydroxy-3,5-diiodophenyl)-3,5-diiodo-L-tyrosine. Its chemical formula is C15H11I4NO4, and its molecular weight is approximately 776.87 g/mol.
Levothyroxine directly influences DNA transcription to increase body metabolism by increasing gluconeogenesis, protein synthesis, the mobilization of glycogen stores, and other functions.
Levothyroxine impurities refer to unintended substances that may be present in Levothyroxine formulations. They can arise during the manufacturing process1 or storage of the medication. It is crucial to monitor and control impurity levels in Levothyroxine as they can affect the drug’s purity, potency, and safety. Stringent quality control measures help minimize impurity levels and ensure the reliability and efficacy of Levothyroxine for the treatment of hypothyroidism. By maintaining strict standards, pharmaceutical manufacturers strive to provide patients with high-quality Levothyroxine formulations that meet regulatory requirements and deliver the intended therapeutic benefits.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for the preparation of Levothyroxine impurity standards, which include 3,3′,5-Triiodothyroacetic acid, 3, 5- Diiodo- L- thyronine, 3,5-diiodo-L-tyrosine, Levothyroxine Impurity F, Levothyroxine Impurity-J, Levothyroxine-N-Lactoside, T4-Aldehyde (Impurity-I), and more. In addition, we offer deuterium-labeled Levothyroxine standards like Levothyroxine-D3, which is essential for conducting bioanalytical research and BA/BE studies. Our Levothyroxine impurity standards have a detailed Certificate of Analysis (CoA) that provides a comprehensive characterization report. This report includes data obtained through techniques,1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. Moreover, we can synthesize unknown Levothyroxine impurity standards, degradation products, and labeled compounds to evaluate the effectiveness of generic Levothyroxine. Each delivery has a comprehensive characterization report.
Higher levels of impurities in Levothyroxine may impact its therapeutic response, efficacy, or consistency, necessitating strict control measures during manufacturing.
Some Levothyroxine impurities may undergo chemical transformations or degradation during storage, which can affect the overall purity and stability of the drug.
Some impurities may interfere with the pharmacokinetics or pharmacodynamics of Levothyroxine, potentially impacting its interactions with other medicines.
Levothyroxine impurities should be stored at a controlled room temperature, usually between 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.