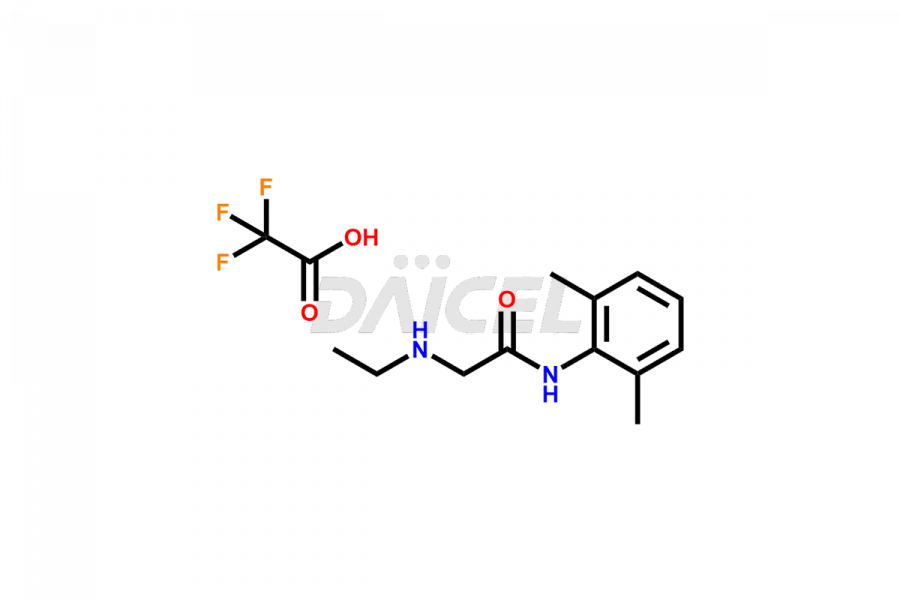
lidocaine
General Information
Lidocaine Impurities and Lidocaine
Daicel Pharma offers the best quality Lidocaine impurities, such as N-desethyl Lidocaine (TFA salt) and N-Nitroso-desethyl lidocaine (Mixture of isomers). It is vital for evaluating the quality, stability, and biological safety of Lidocaine. In addition, Daicel Pharma specializes in the custom synthesis of Lidocaine impurities and ensures their worldwide delivery.
Lidocaine [CAS: 137-58-6], or Lignocaine, is a tertiary amine and a local anesthetic agent. It also has analgesic and antiarrhythmic uses. Lidocaine with vasopressors aids in local anesthesia. It helps in advanced airway management during tracheal intubation.
Lidocaine: Use and Commercial Availability
Lidocaine has various routes of administration. Lidocaine is available as gels, ointments, intravenous solutions, and more. As a local anesthetic, it numbs tissue sensations during surgeries. Lidocaine is a part of the World Health Organization’s List of Essential Medicines. Lidocaine and other medicines treat neuropathic pain. It has wide use in facial plastic surgery. Lidocaine is available under different generic names. Xylocaine, Alphacaine, Anestacon, Dentipatch, Glydo, Lidoderm, etc., are some brands under which Lidocaine. Lidocaine is also available in combination with other medicines.
Lidocaine Structure and Mechanism of Action


The chemical name of Lidocaine is 2-(Diethylamino)-N-(2,6-dimethylphenyl)acetamide. The chemical formula for Lidocaine is C14H22N2O, and its molecular weight is approximately 234.34 g/mol.
Lidocaine stabilizes the neuronal membrane. It inhibits ionic fluxes needed for impulse initiation and conduction, thus causing local anesthetic action.
Lidocaine Impurities and Synthesis
Impurities may occur during the synthesis of Lidocaine 1 that affect drug safety and efficacy. They form during the synthesis, storage, or degradation of Lidocaine. It is necessary to control and monitor the impurities of Lidocaine to improve drug safety, efficacy, and storage.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Lidocaine impurities, which includes N-desethyl Lidocaine (TFA salt) and N-Nitroso-desethyl lidocaine (Mixture of isomers). The CoA is from a cGMP-compliant analytical facility. It contains the complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional data like 13C-DEPT and CHN on request. Daicel Pharma can prepare any unidentified Lidocaine impurity or degradation product. In addition, Daicel Pharma offers a highly purified deuterium-labeled standard of N-Nitroso-desethyl lidocaine-d5 (Mixture of isomers) for bioanalytical research and BA/BE studies. We also provide a complete characterization report on delivery.
References
FAQ's
References
- Lofgren, Nils M.; Lundqvist, Bengt J., Alkyl glycinanilides, US2441498, May 11, 1948, Aktiebolaget Astra Apotekarnes Kemiska Fabriker
- Caille, G.; Lelorier, J.; Latour, Y.; Besner, J. G., GLC determination of lidocaine in human plasma, Journal of Pharmaceutical Sciences, Volume: 66, Issue: 10, Pages: 1383-5, 1977 DOI: (10.1002/jps.2600661007)
Frequently Asked Questions
What are the chief Lidocaine impurities?
2,6-Dimethylaniline (DMA) and Lidocaine hydrochloride monohydrate are chief Lidocaine impurities.
Which is the analytical method to identify Lidocaine degradation-related impurities?
LC-MS is the analytical method to identify Lidocaine degradation-related impurities.
What is the purpose of Lidocaine impurities in the drug industry?
Lidocaine impurities help in pharmaceutical research, method validation, and stability studies.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.