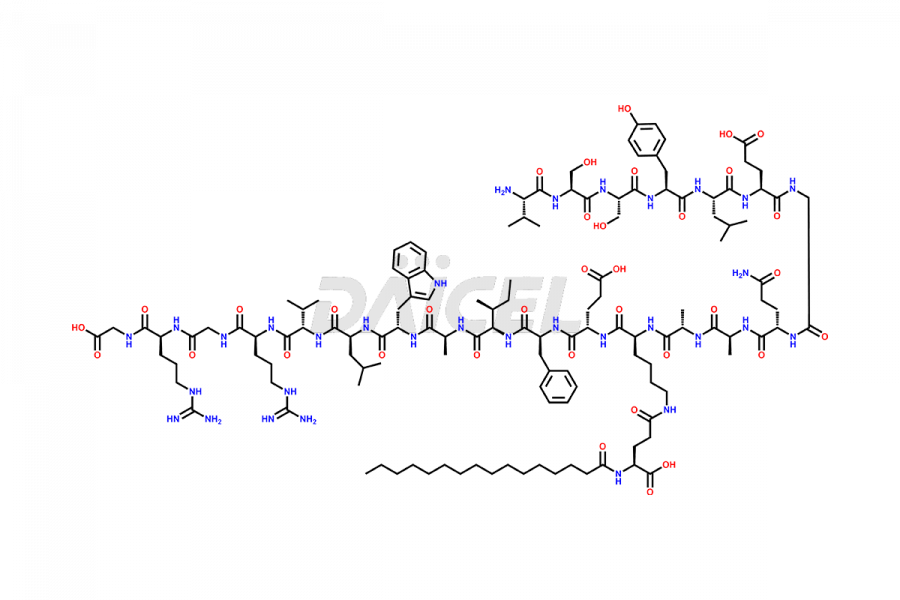
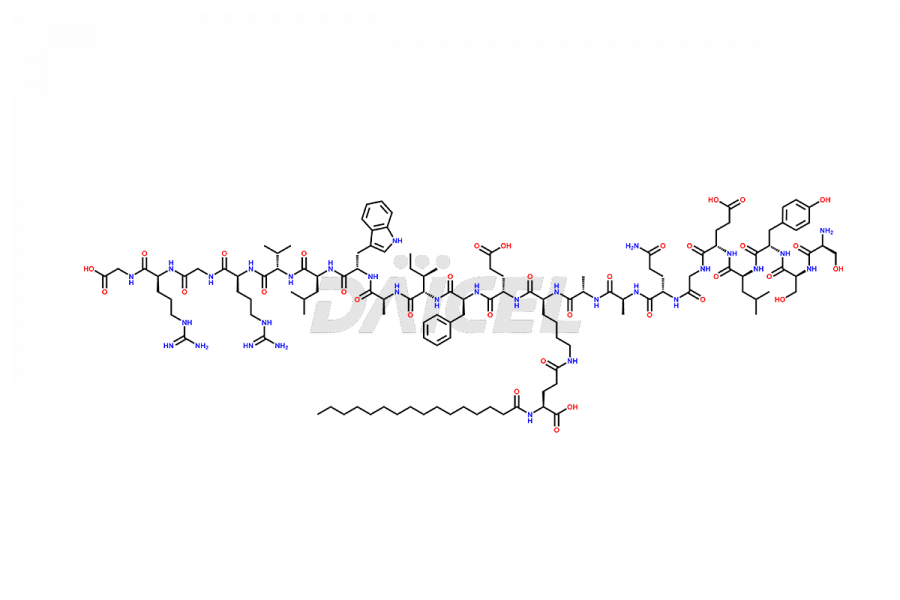
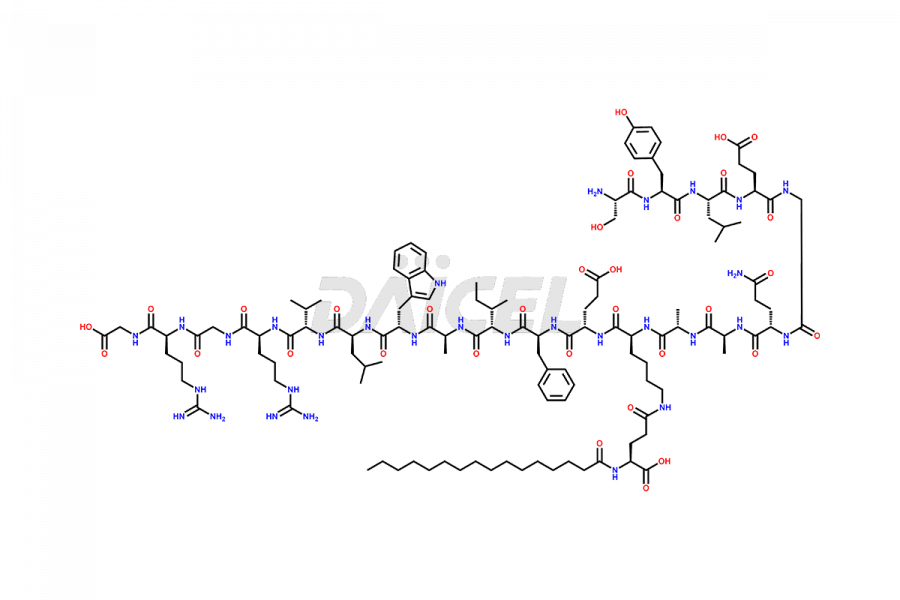
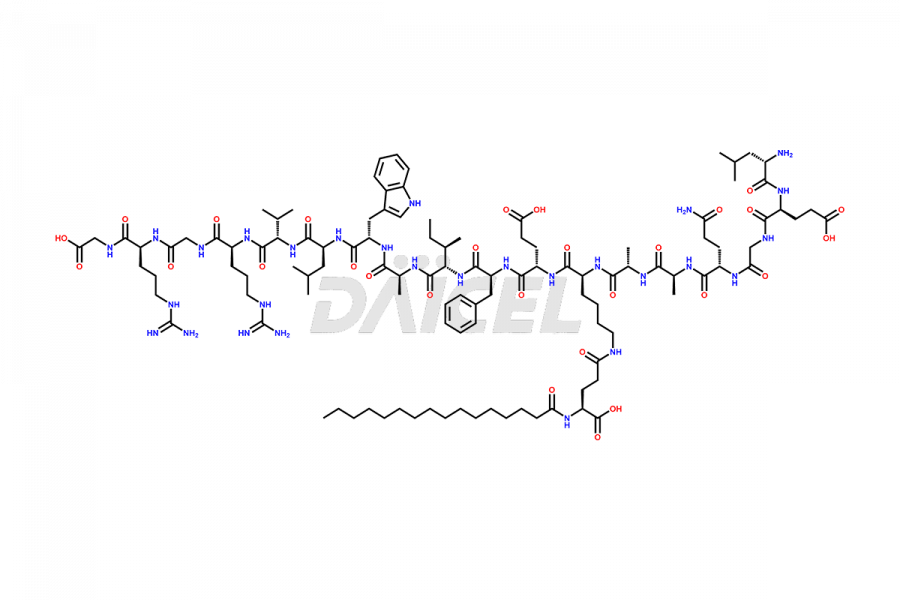
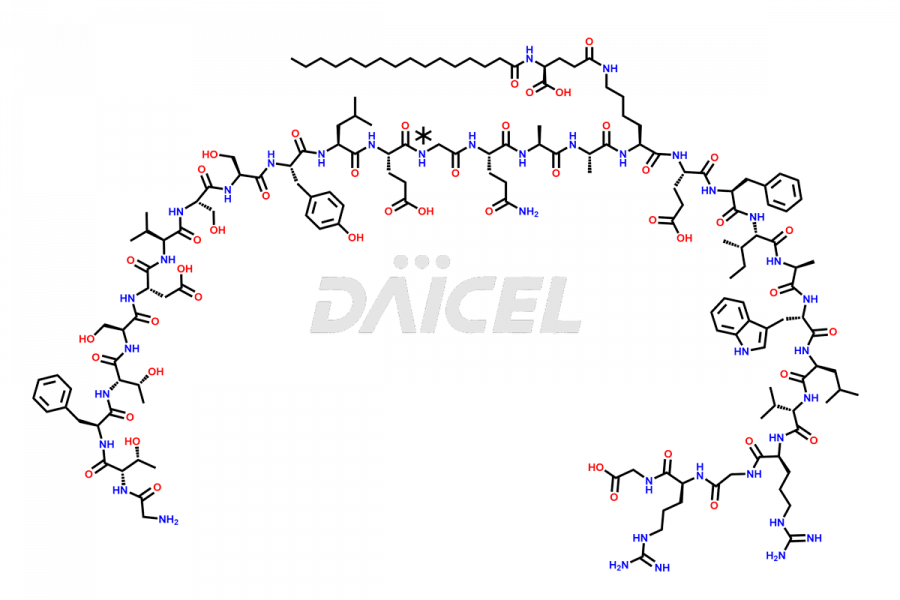
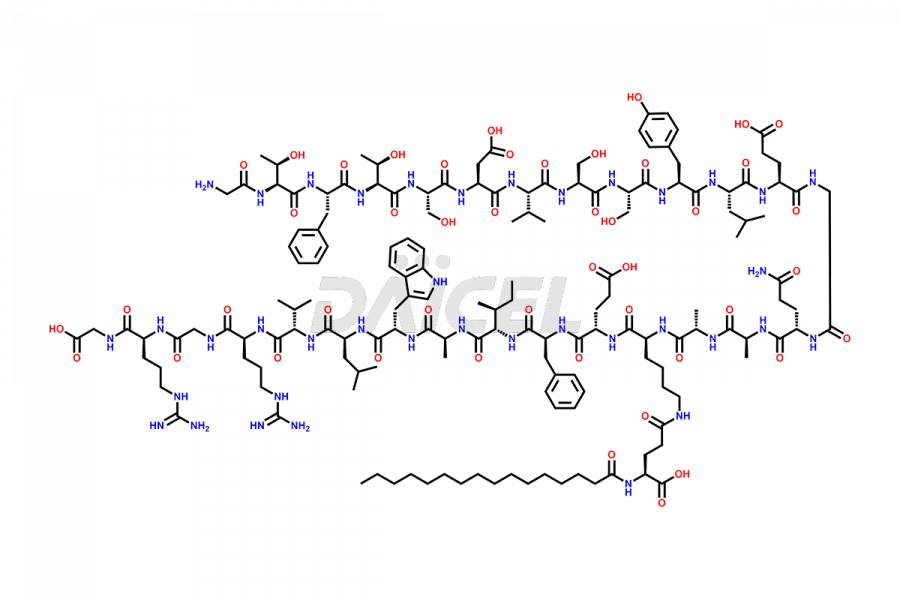
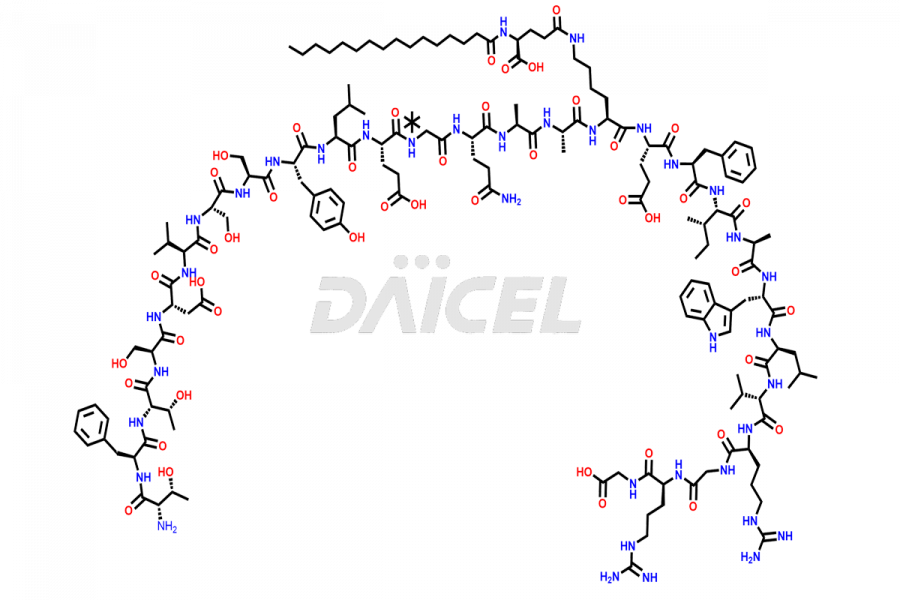
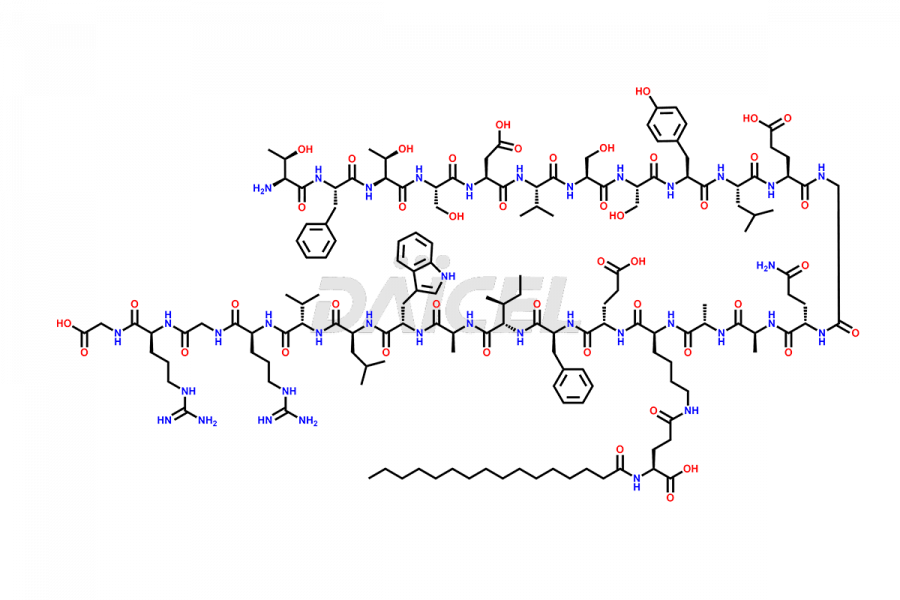
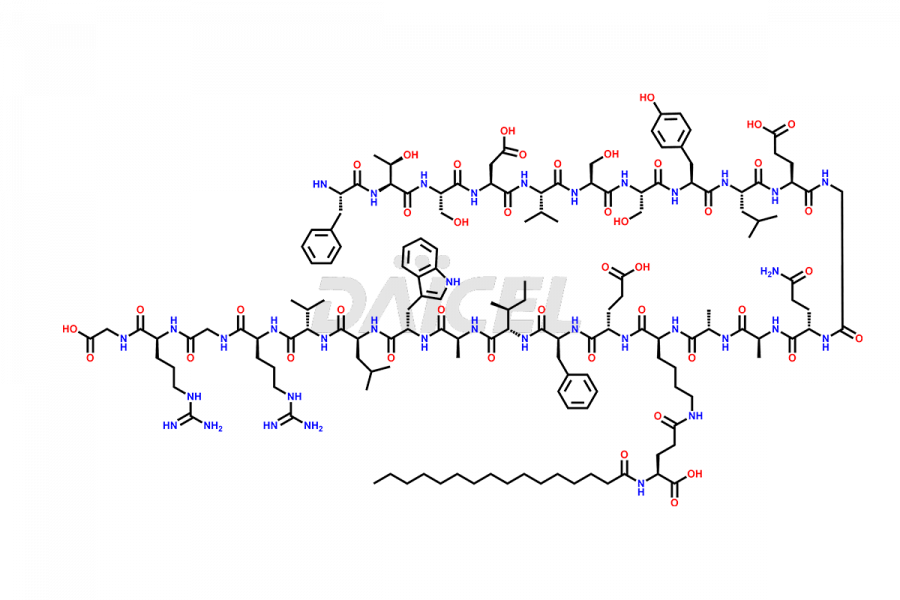
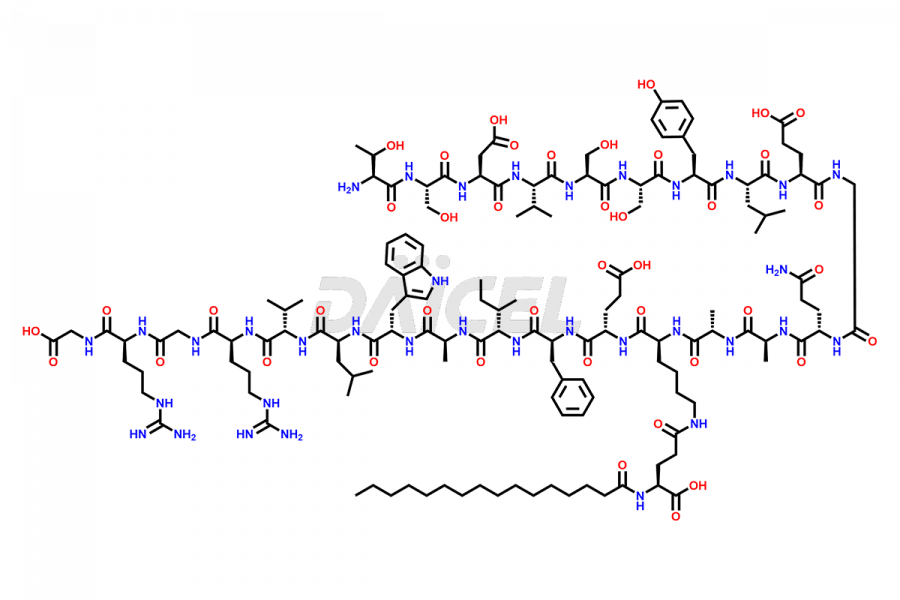
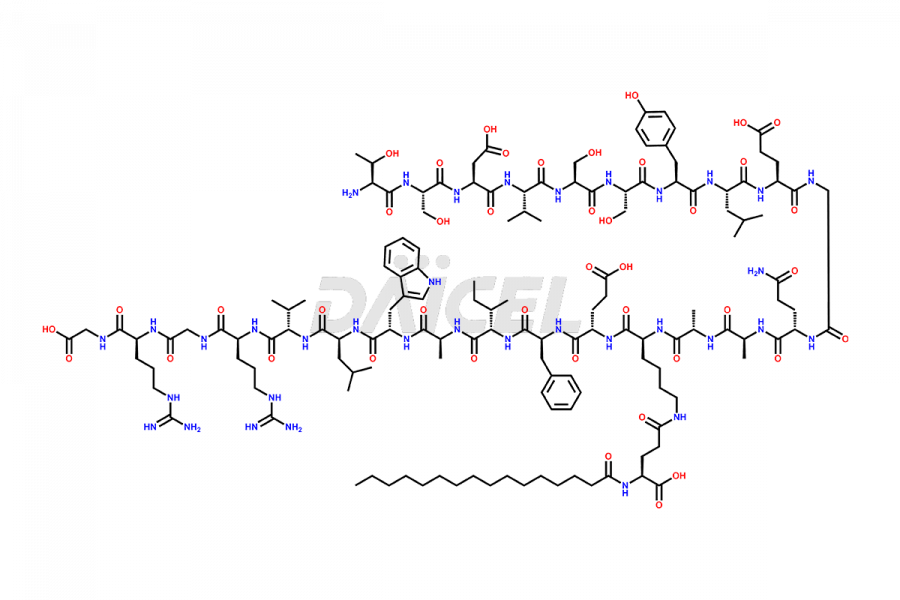
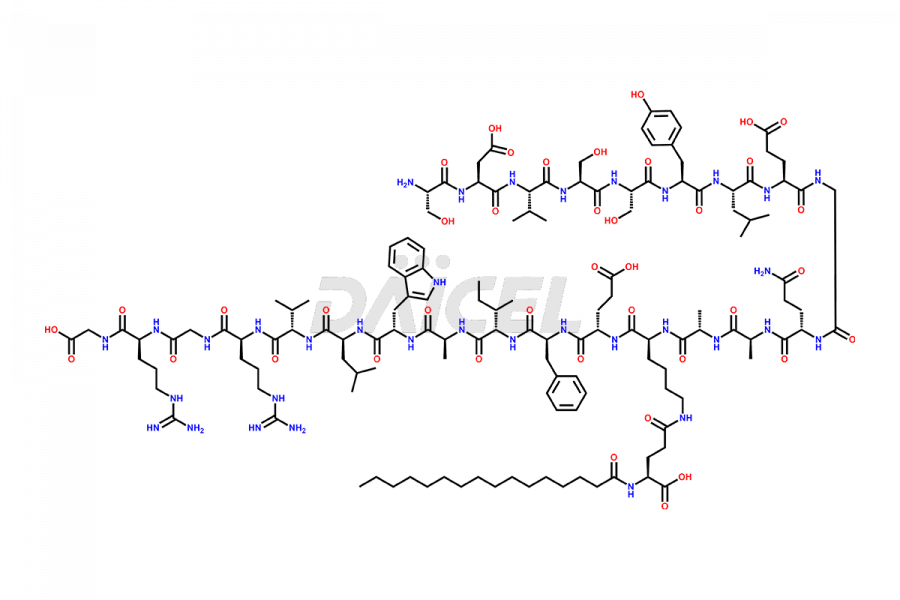
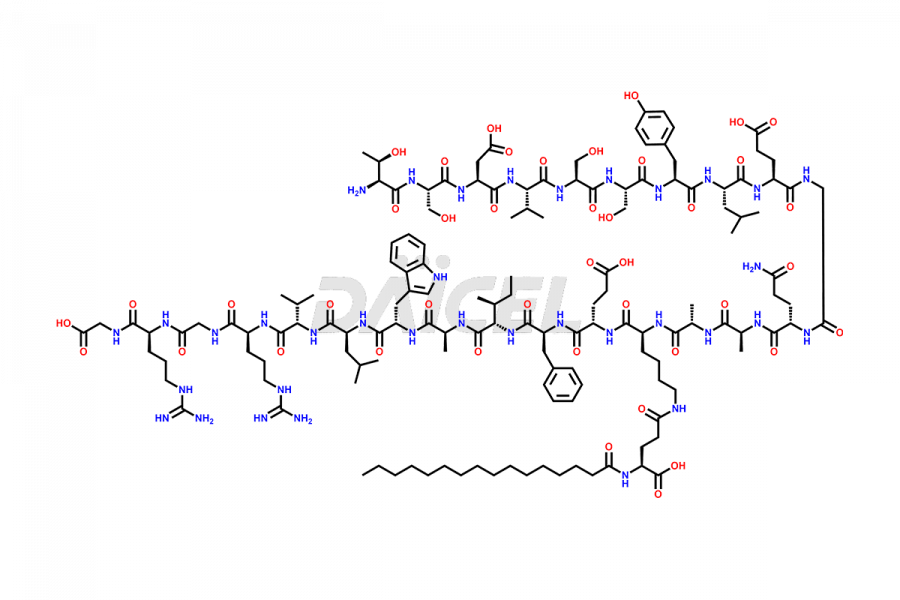
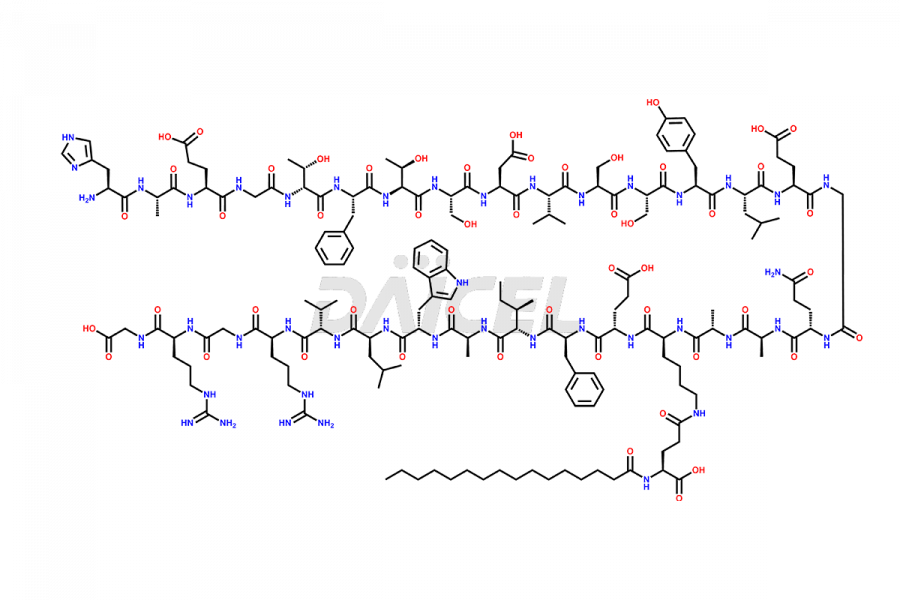
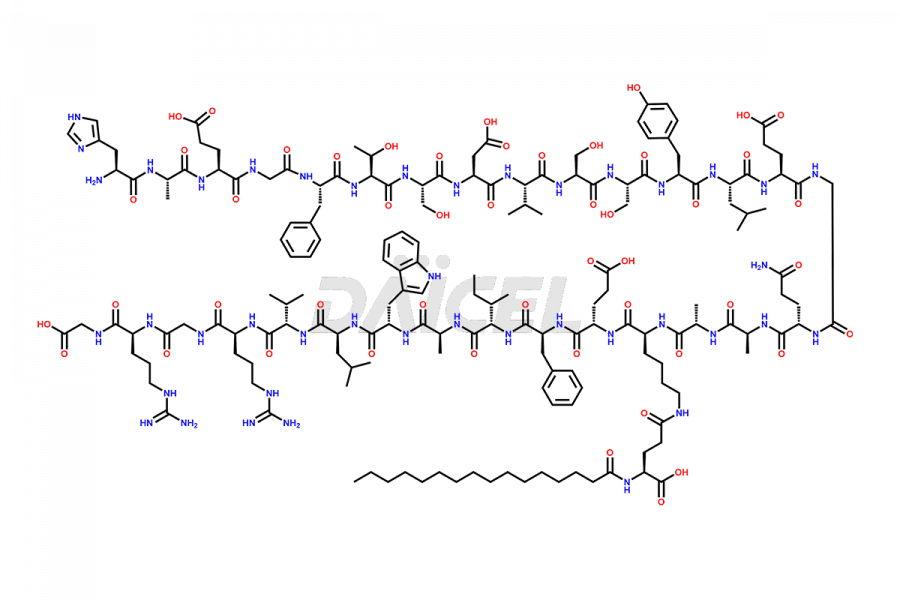
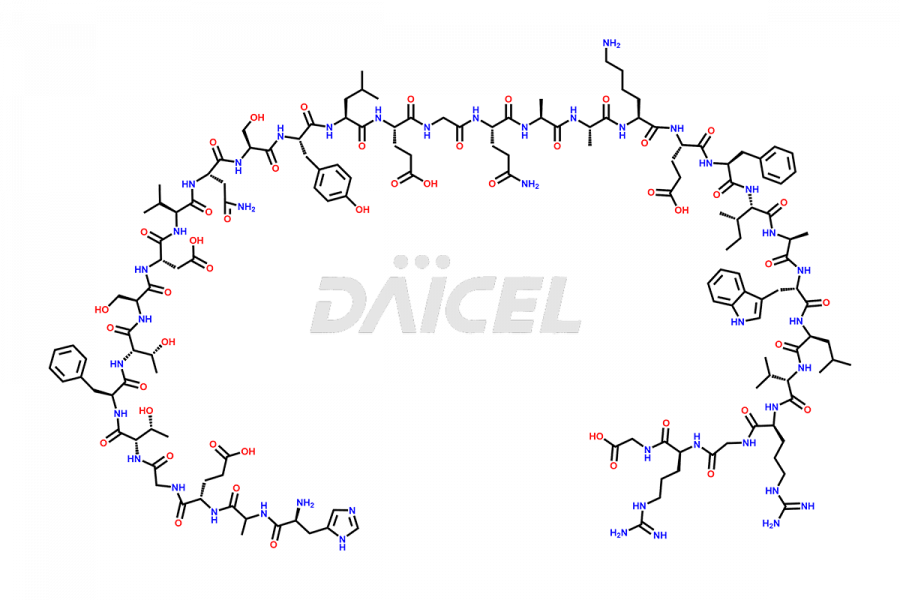
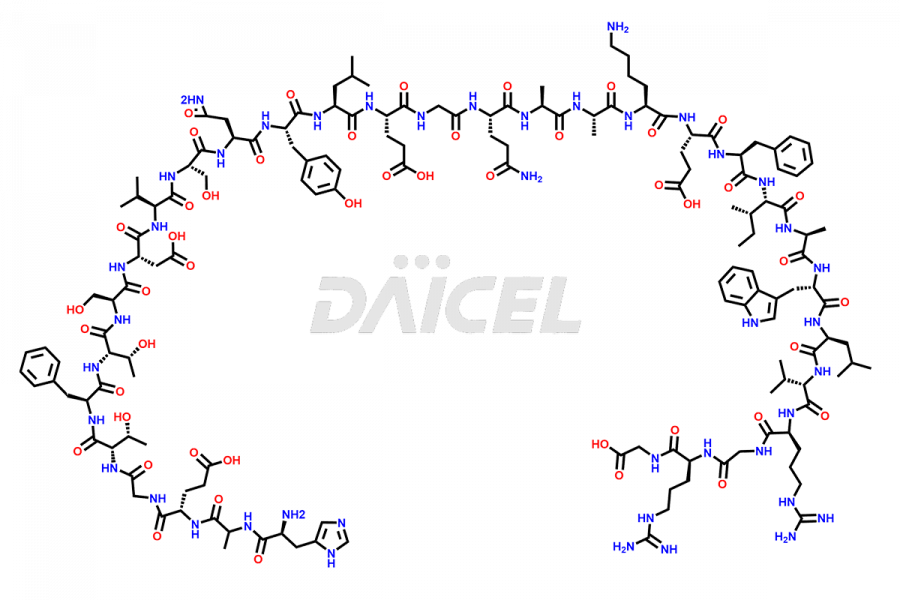
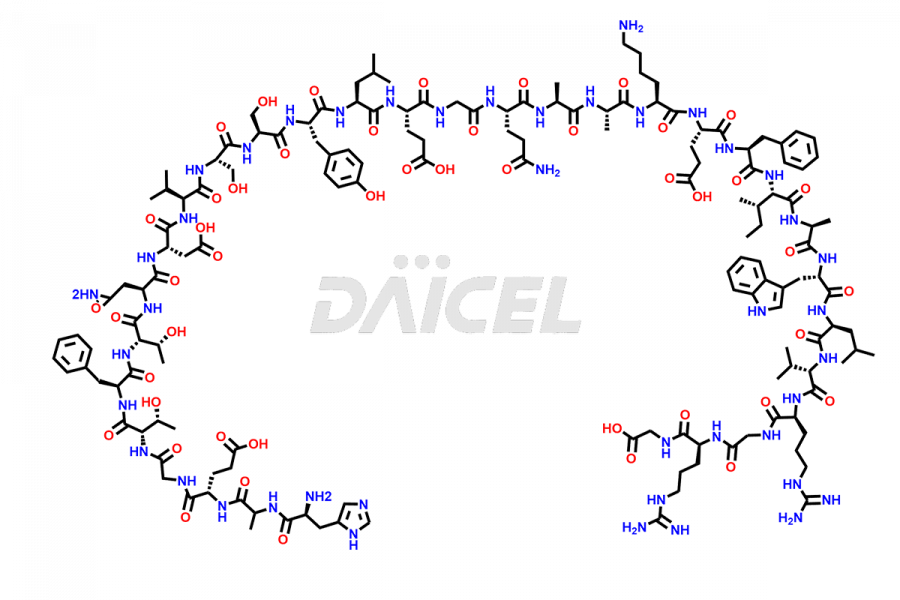
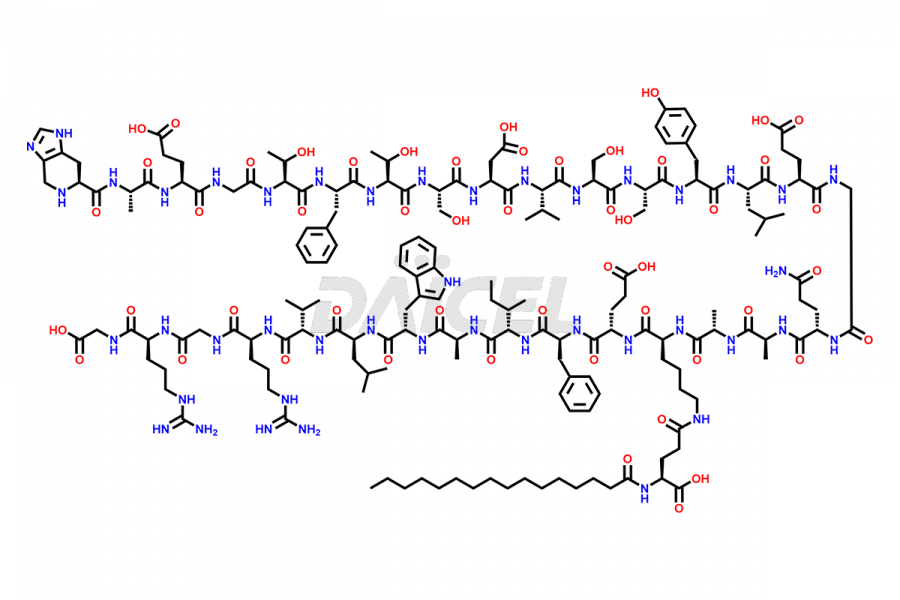
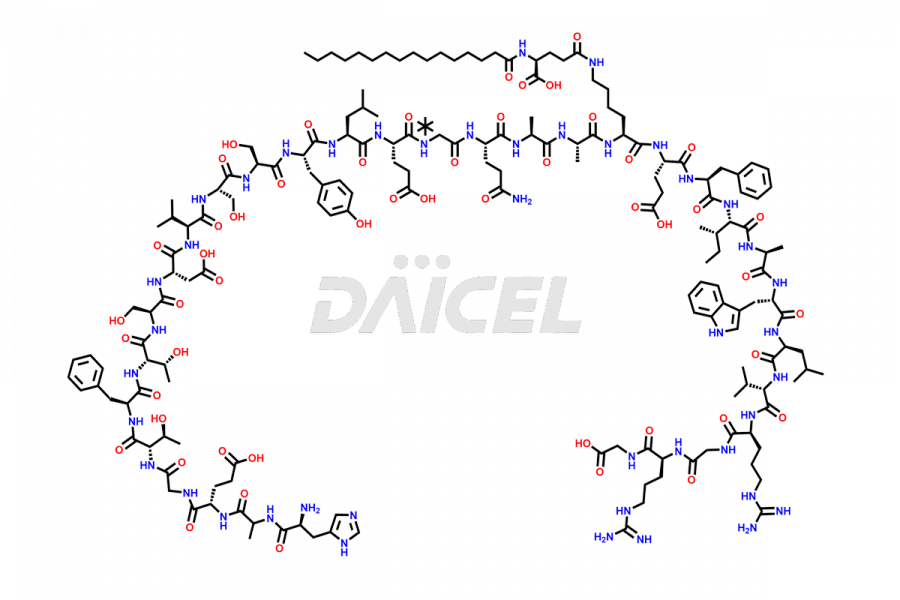
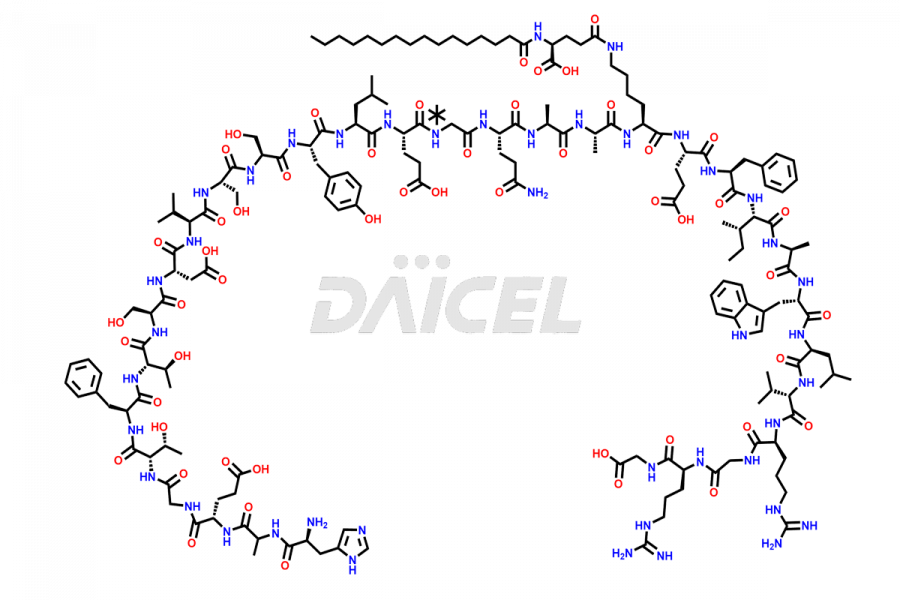
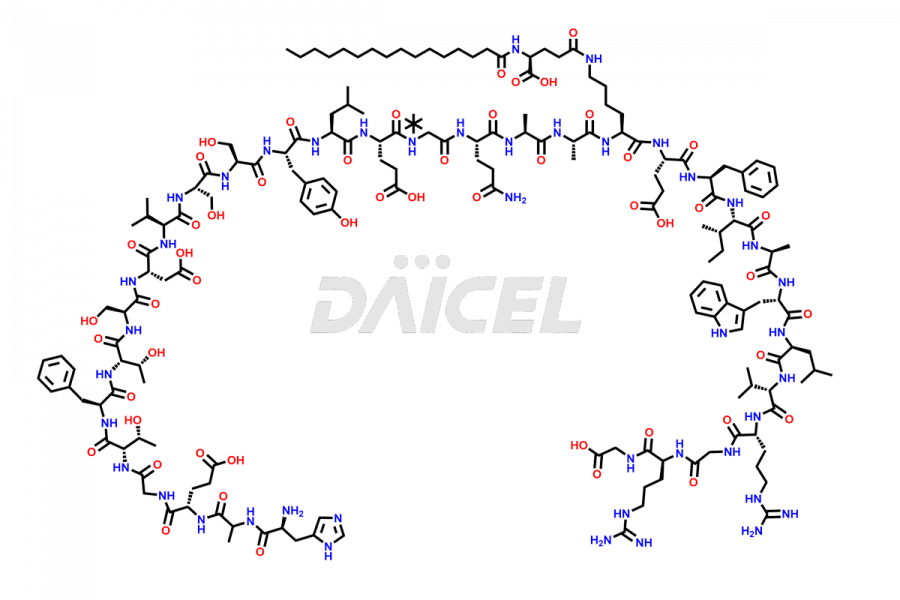
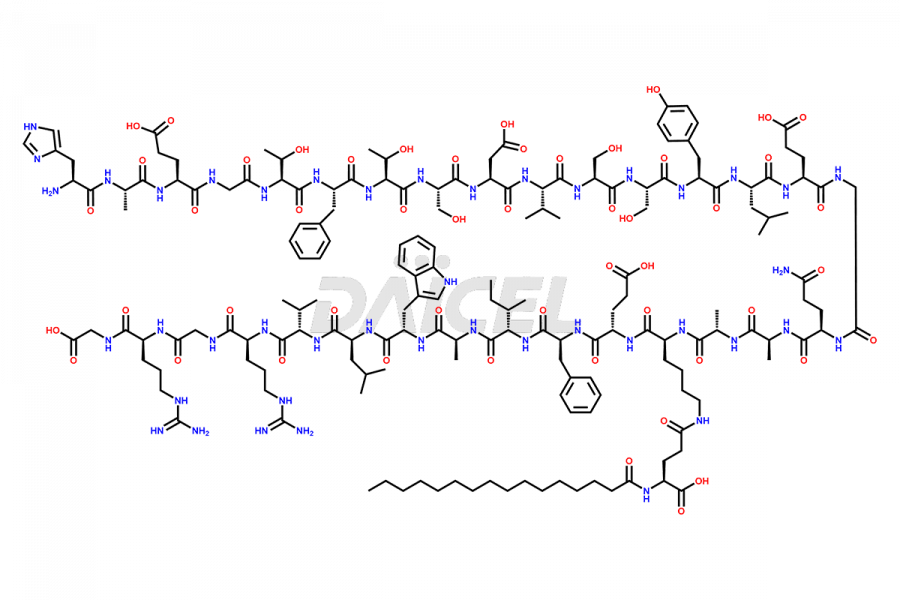
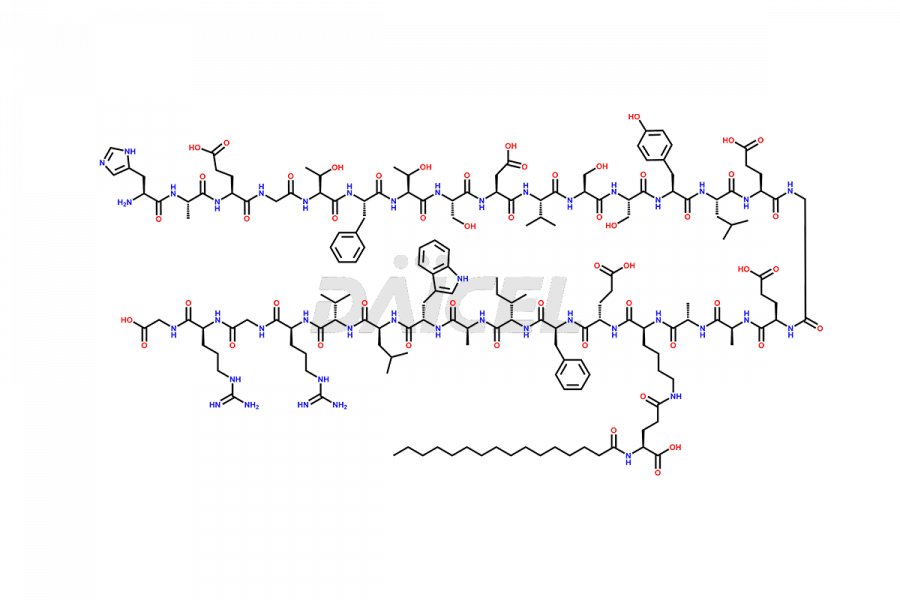
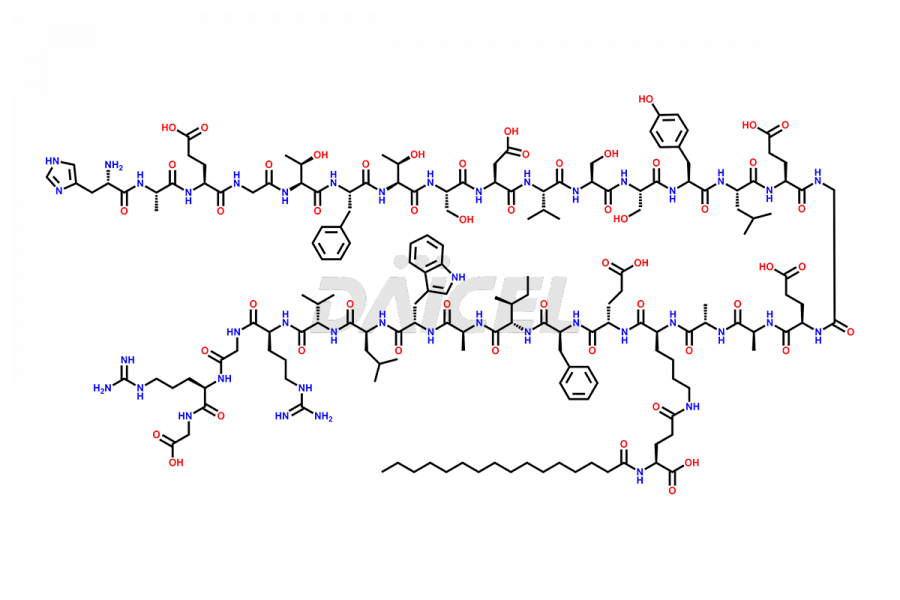
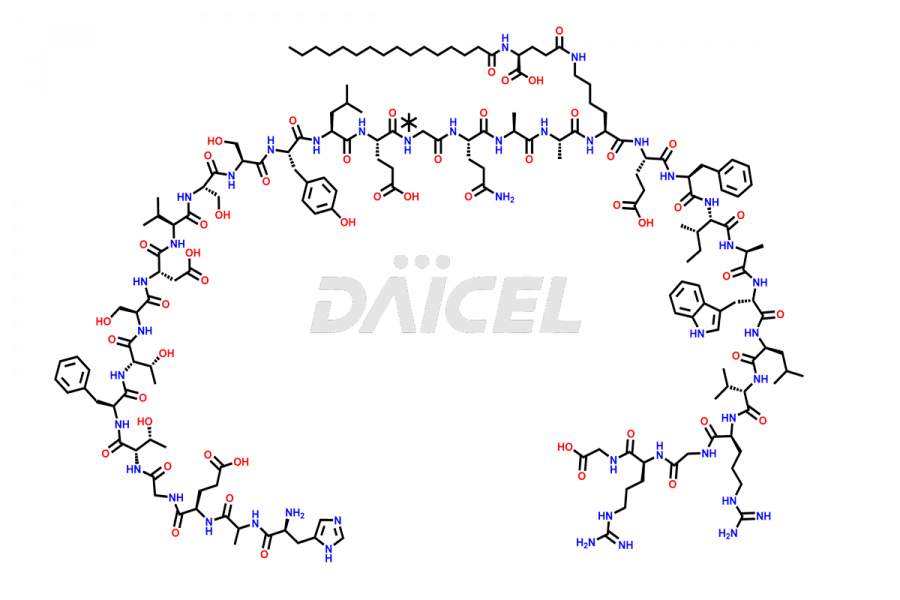
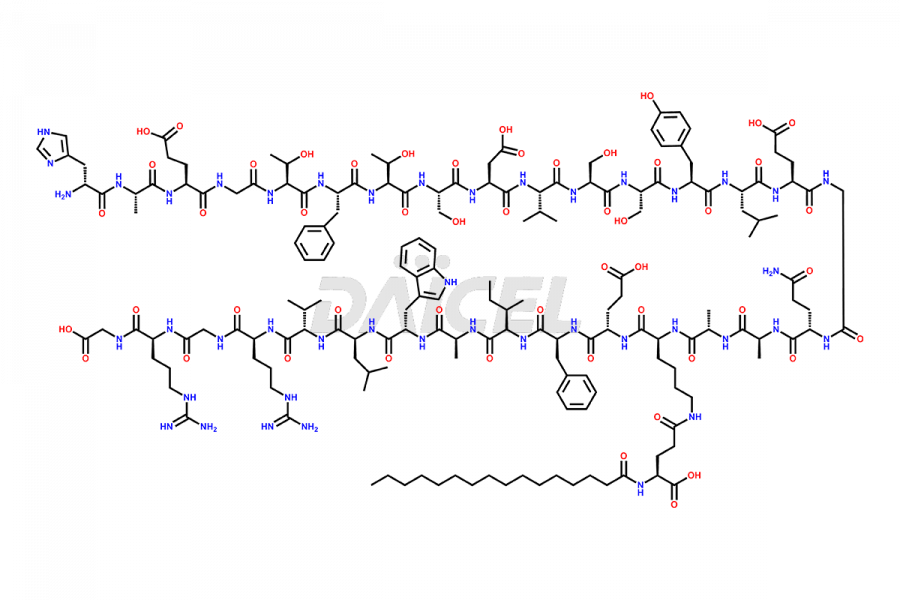
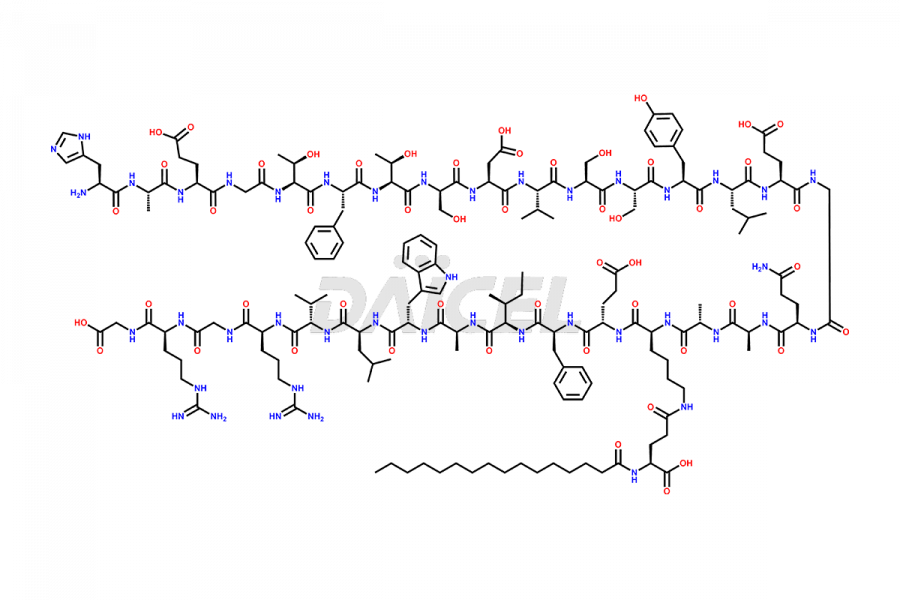
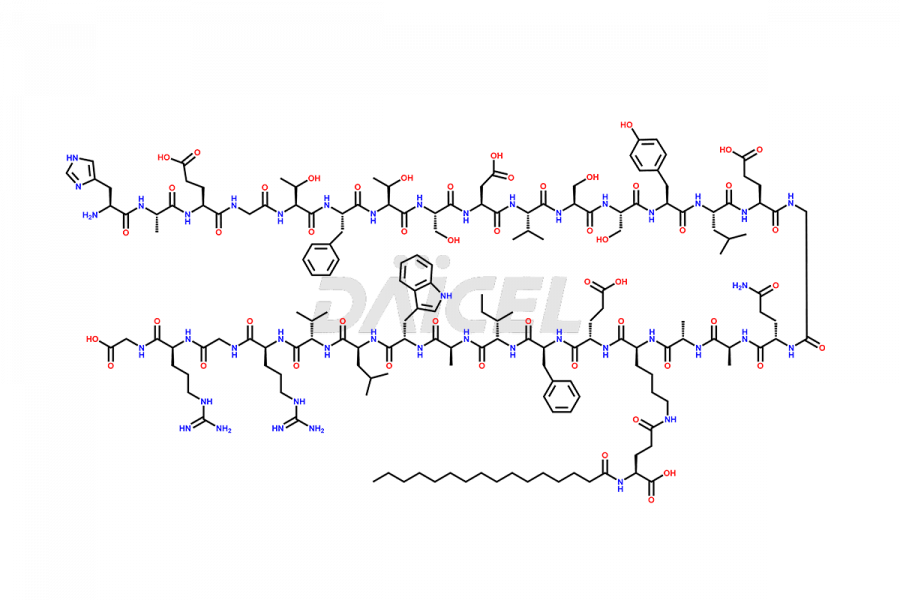
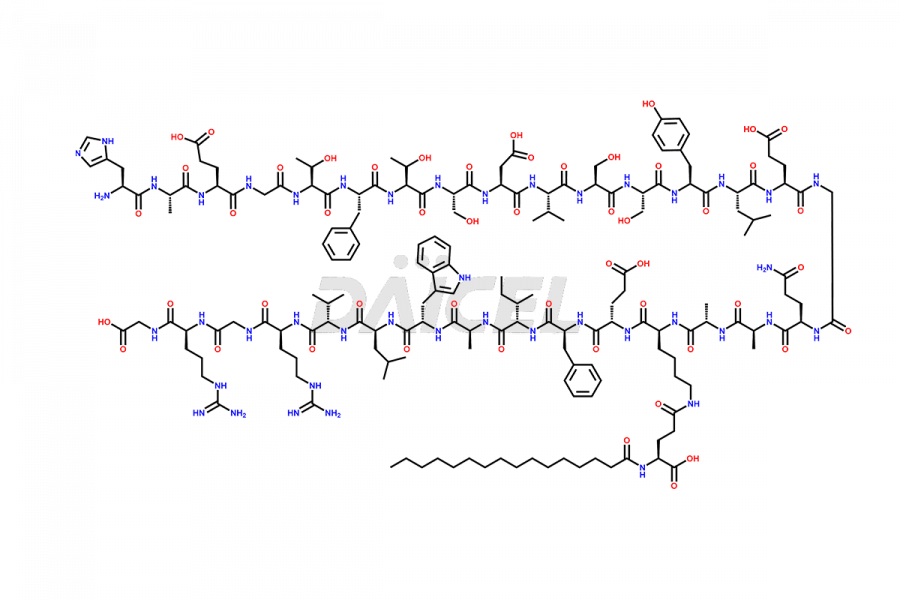
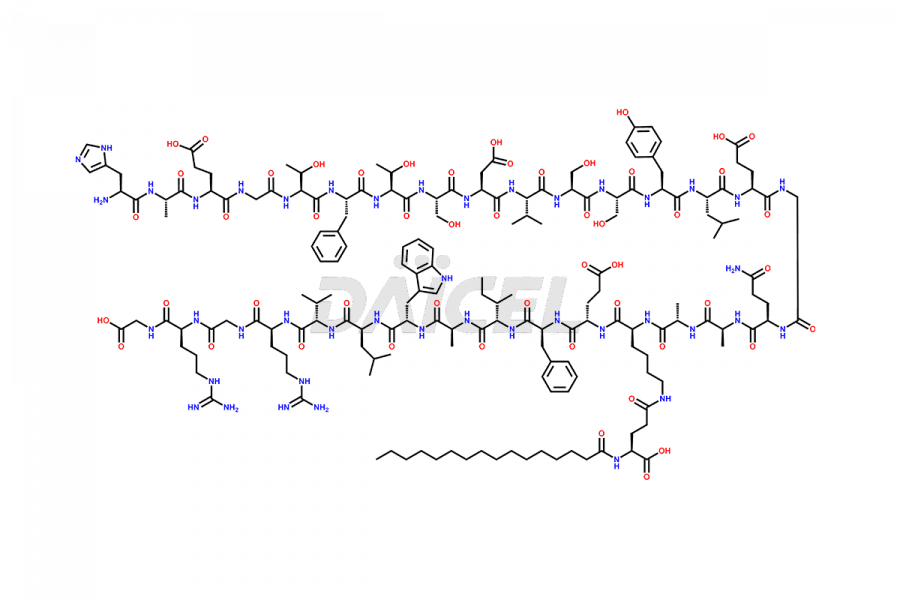
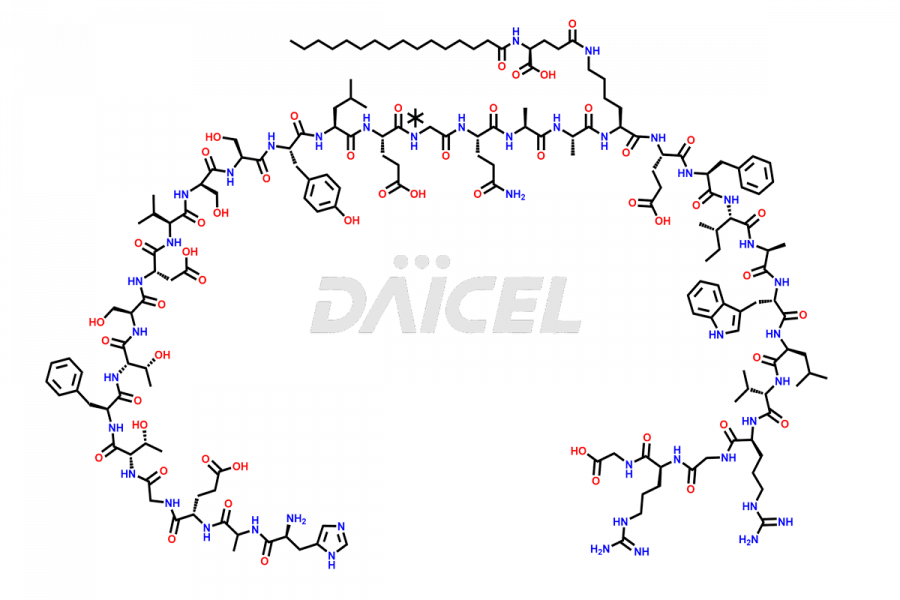
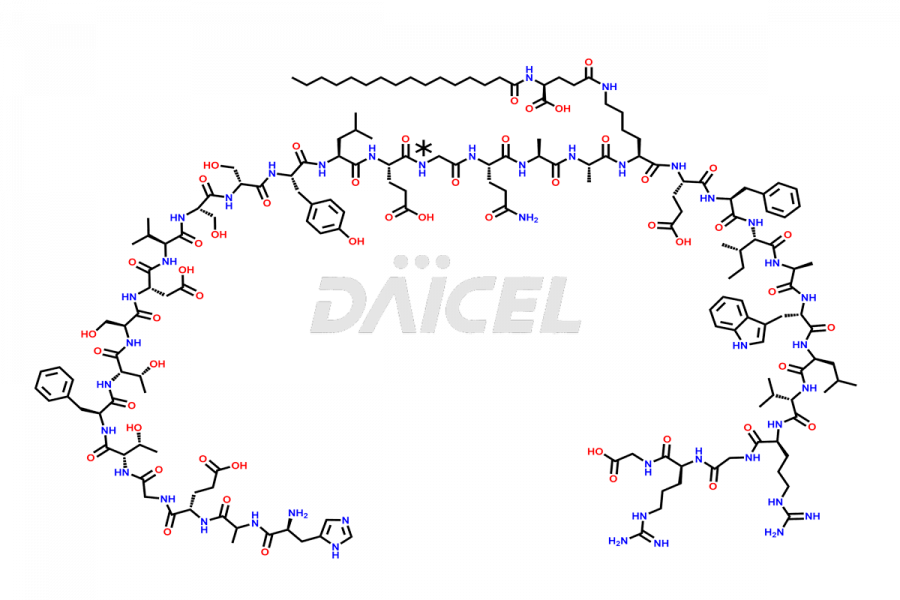
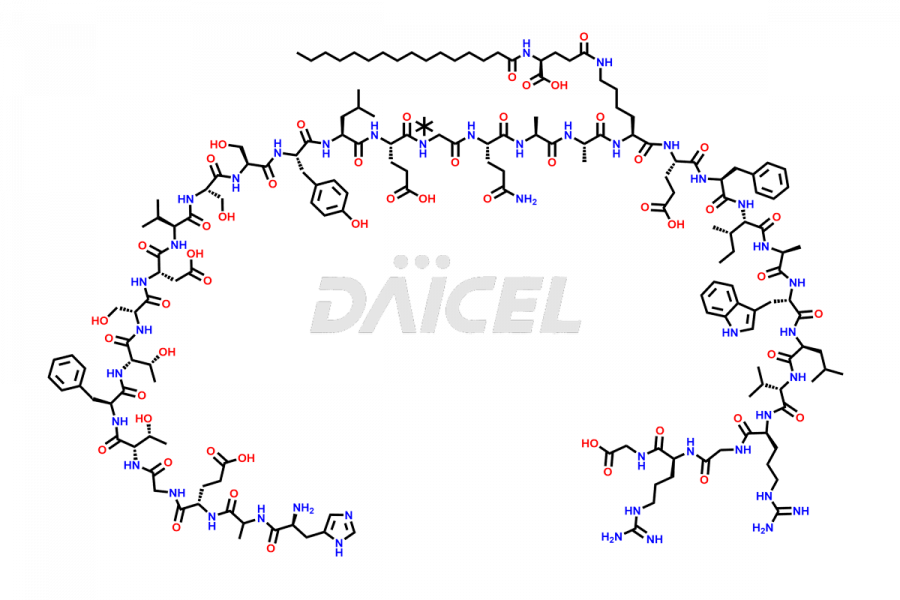
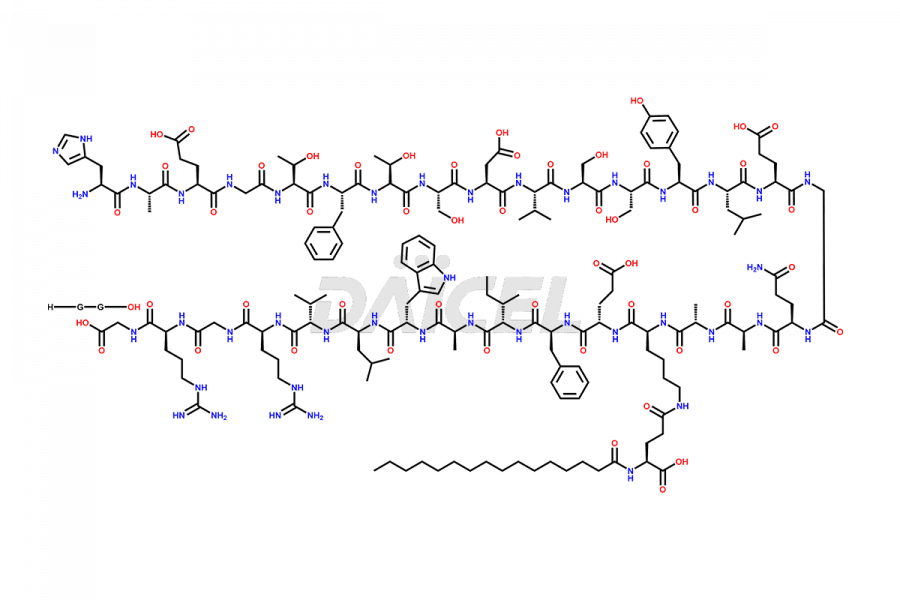
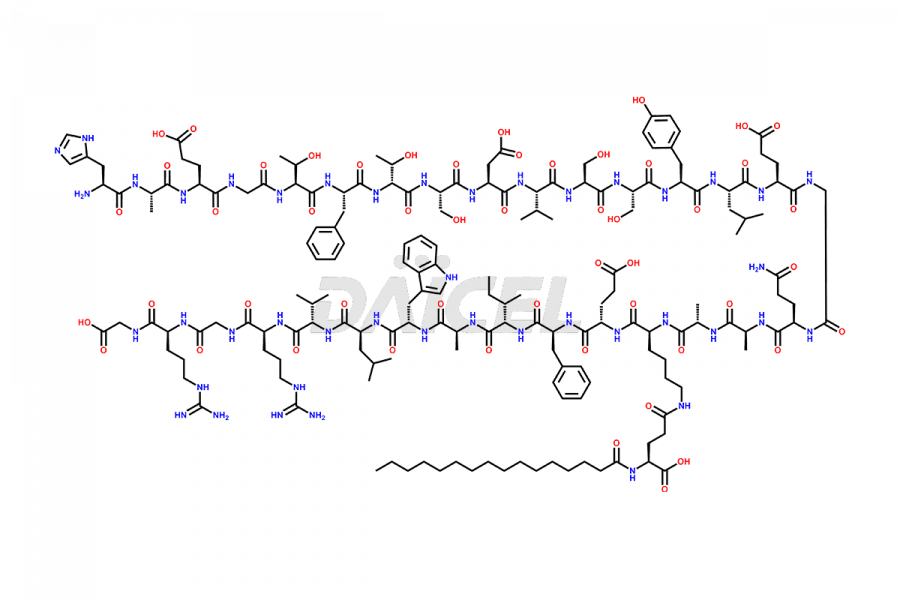
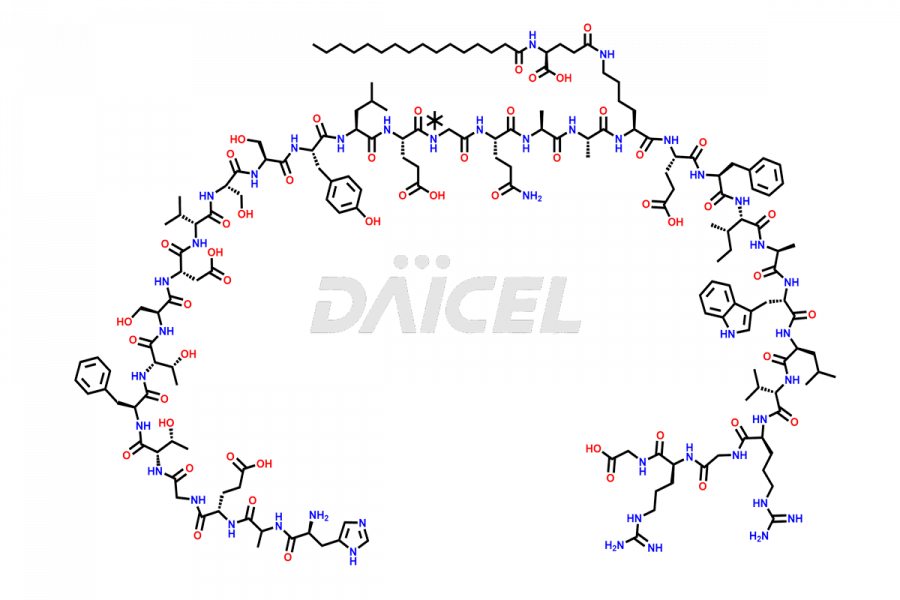
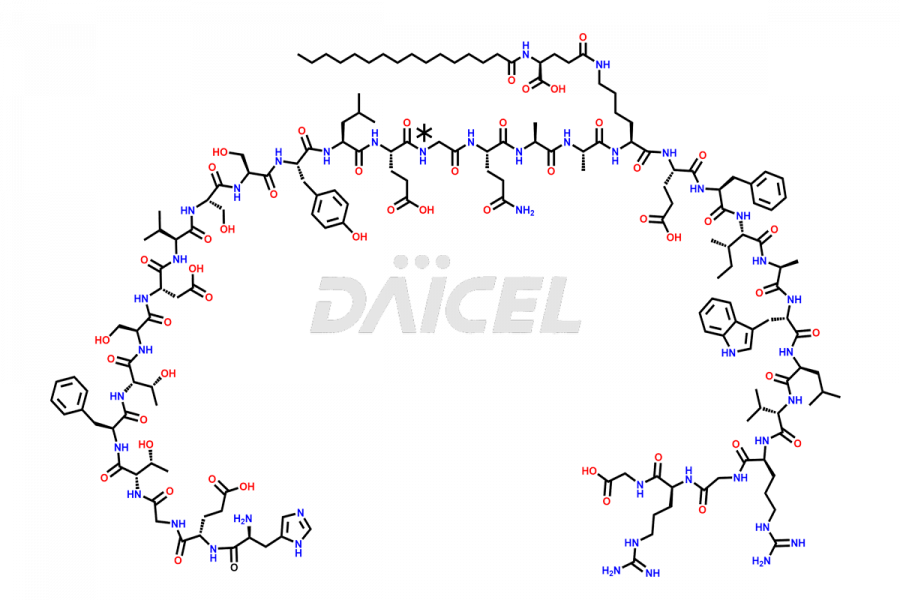
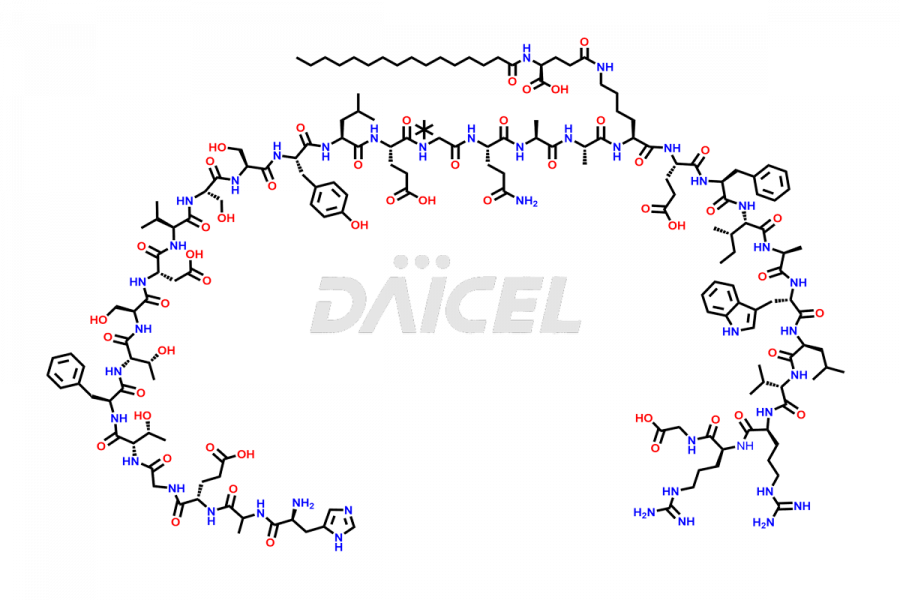
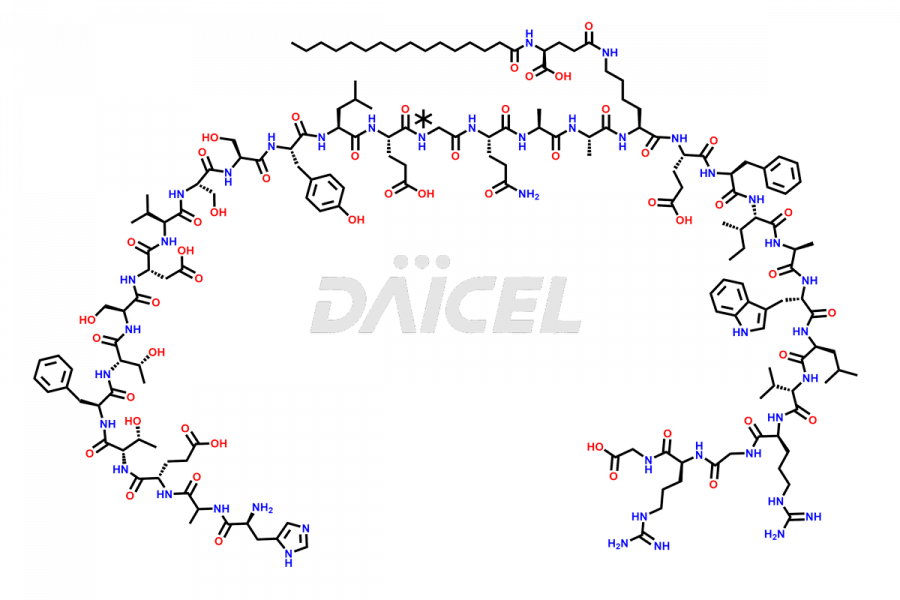
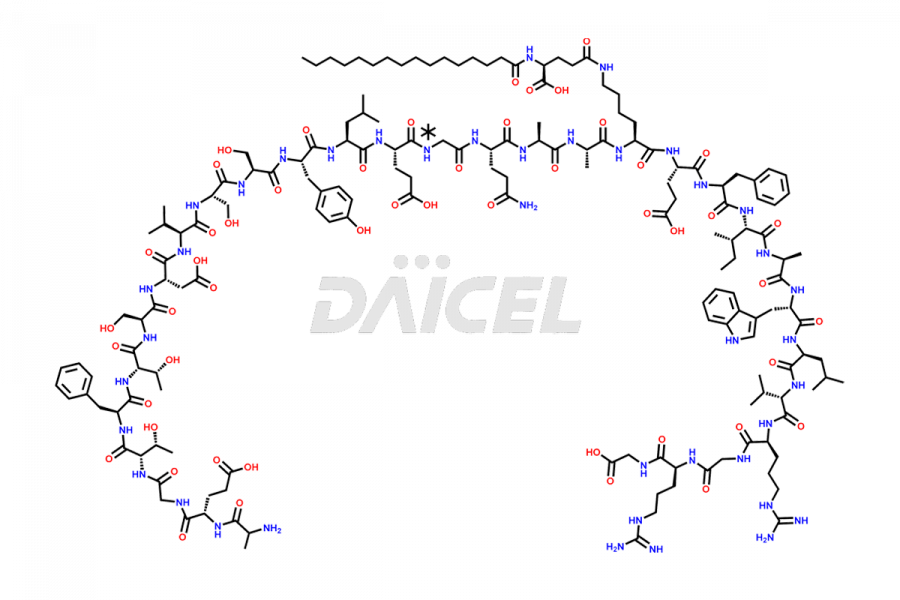
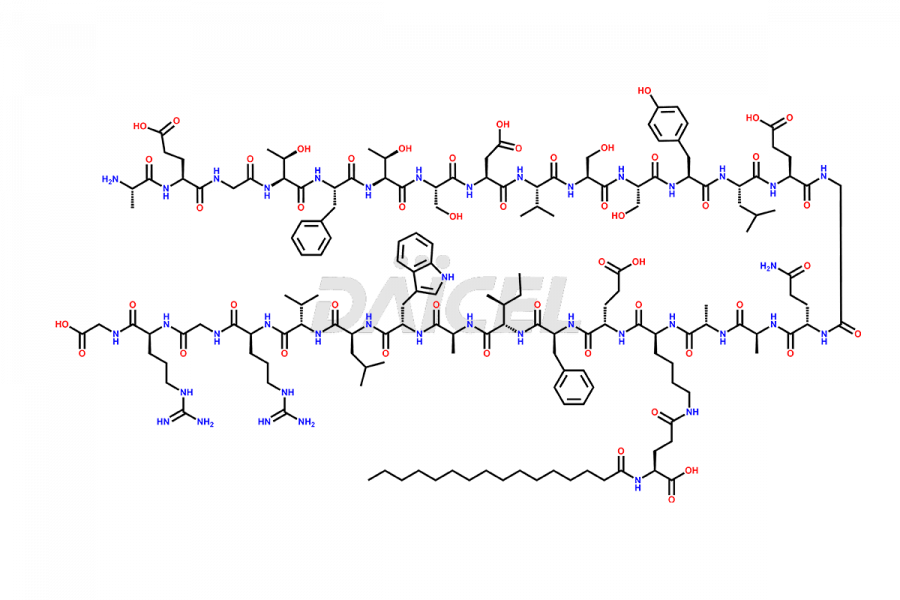
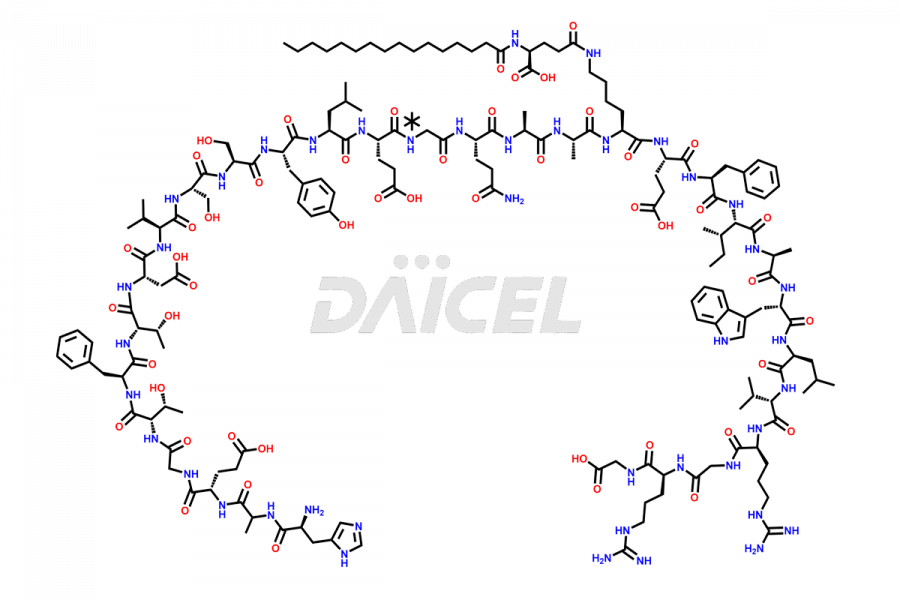
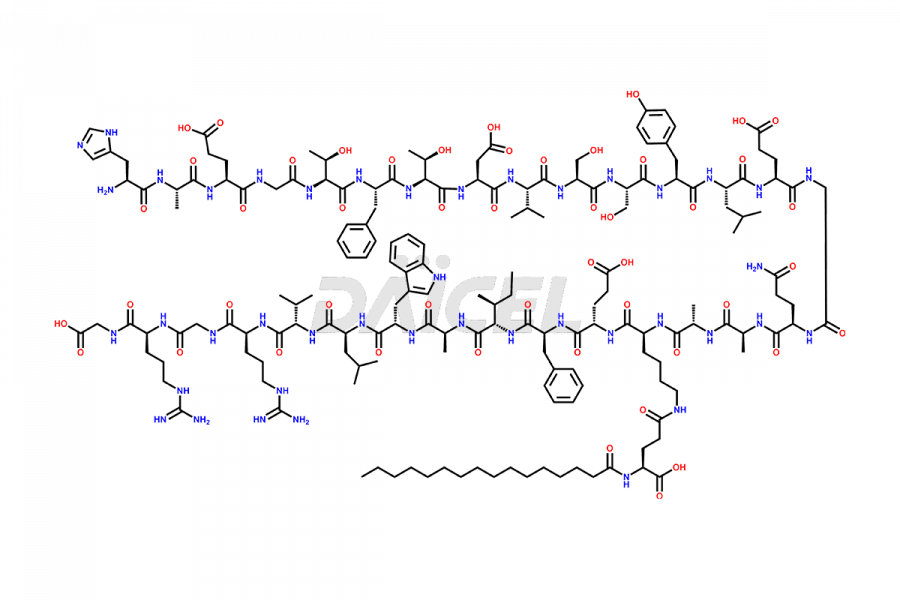
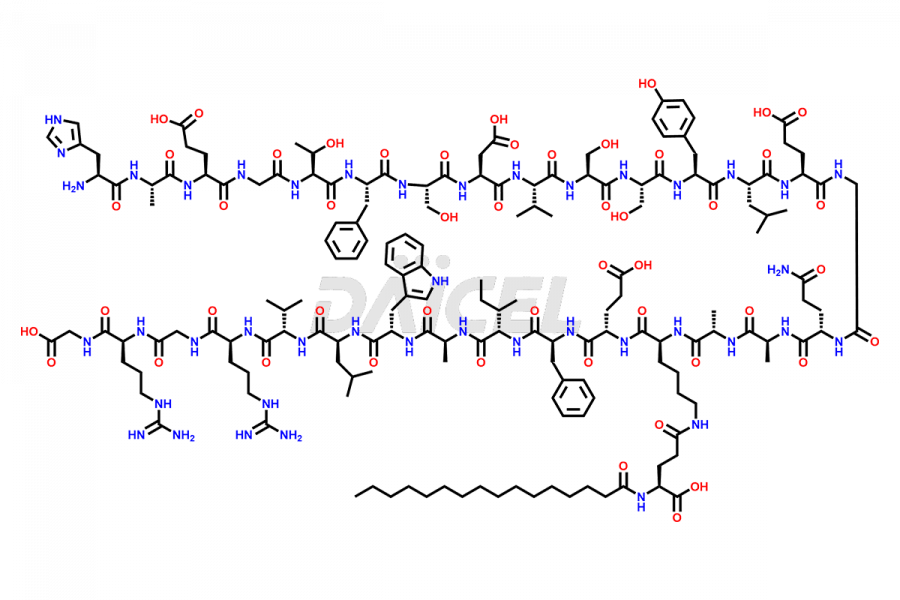
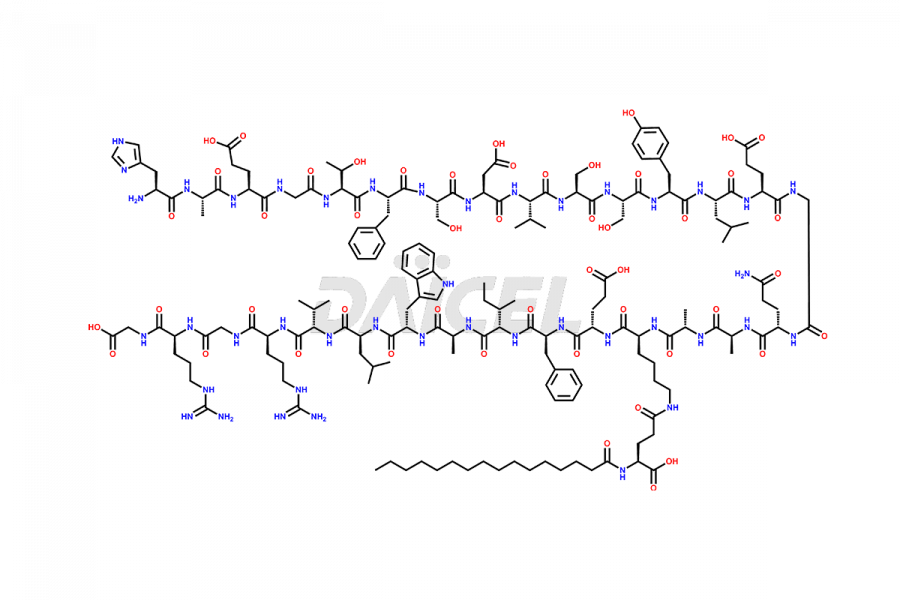
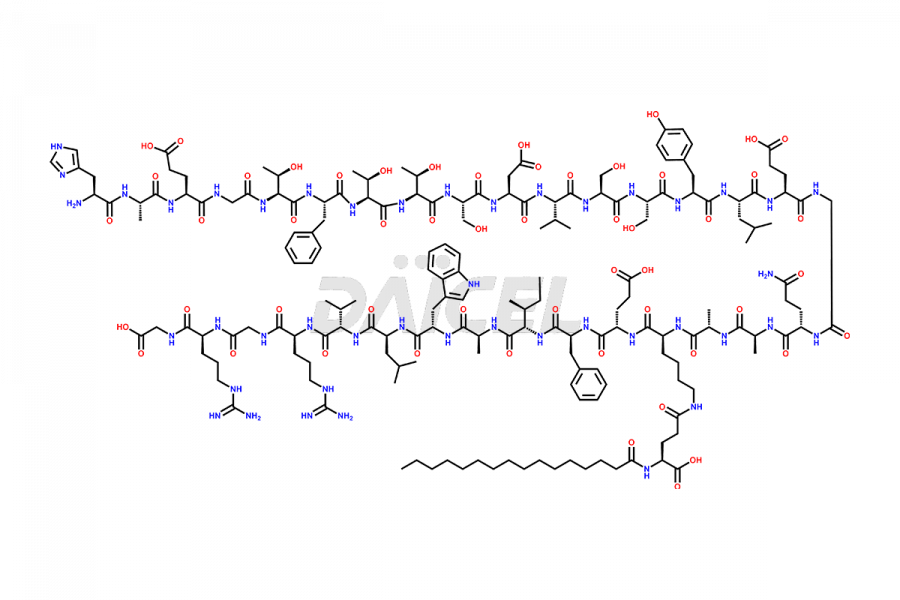
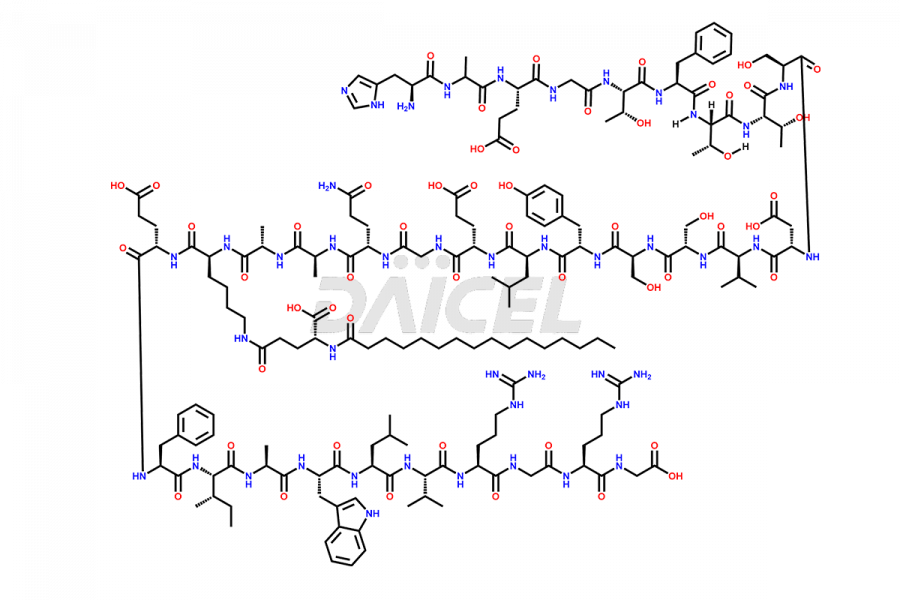
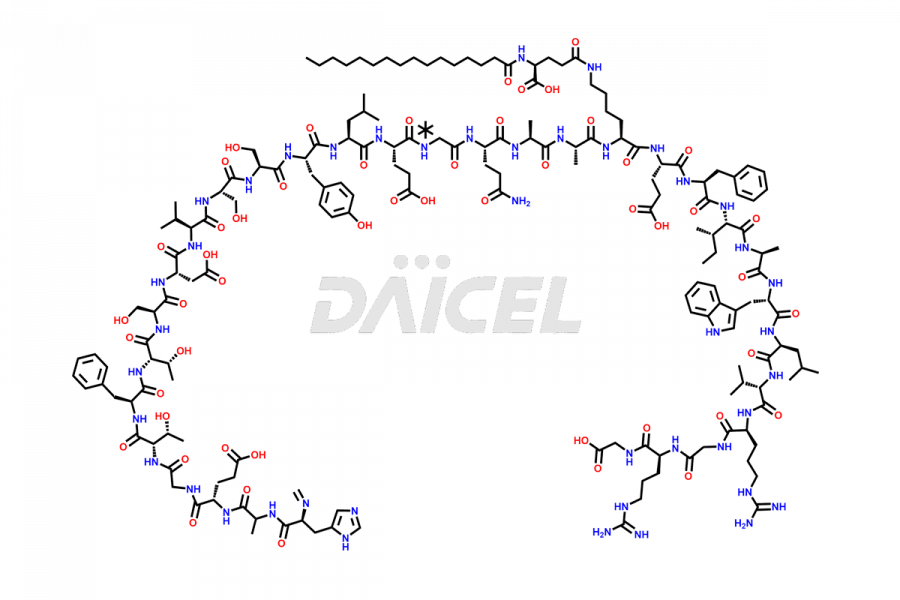
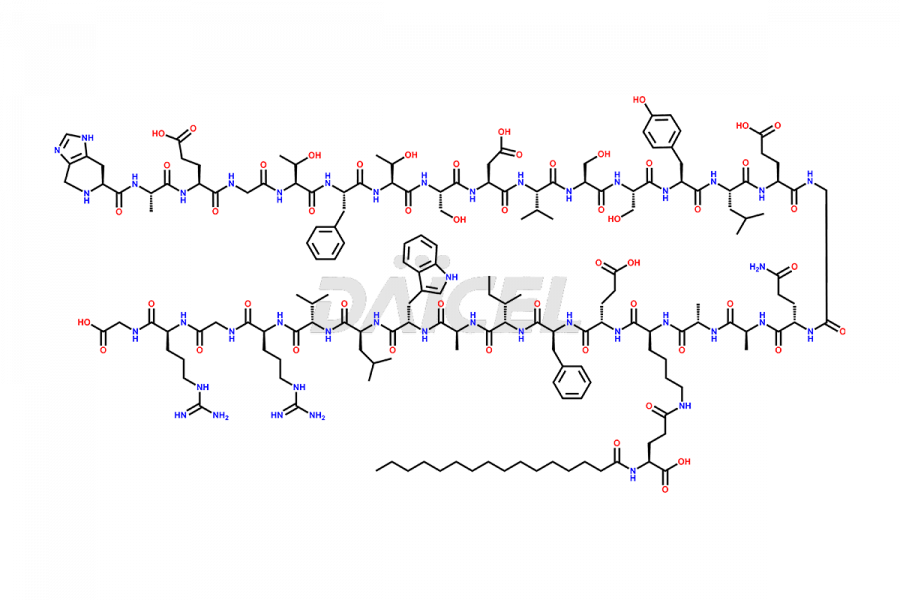
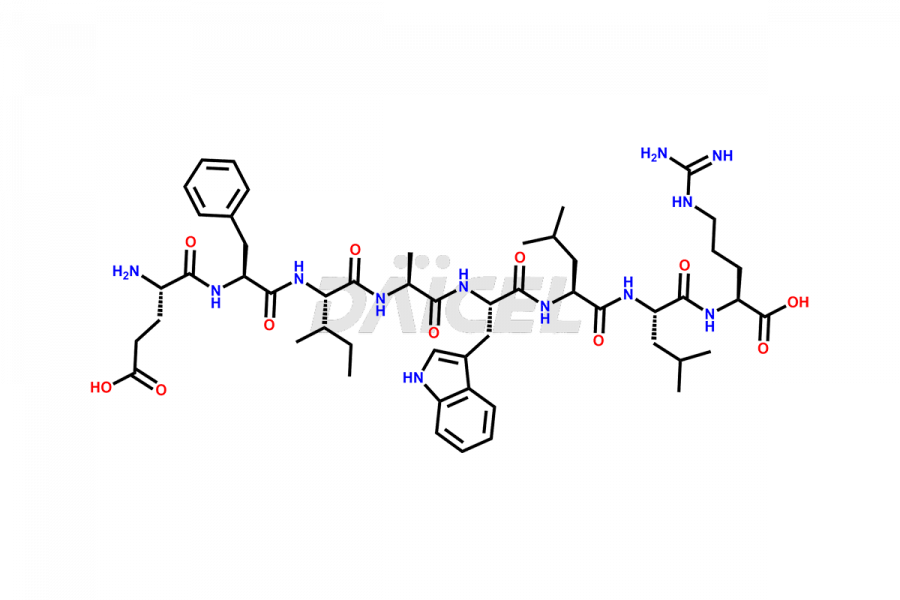
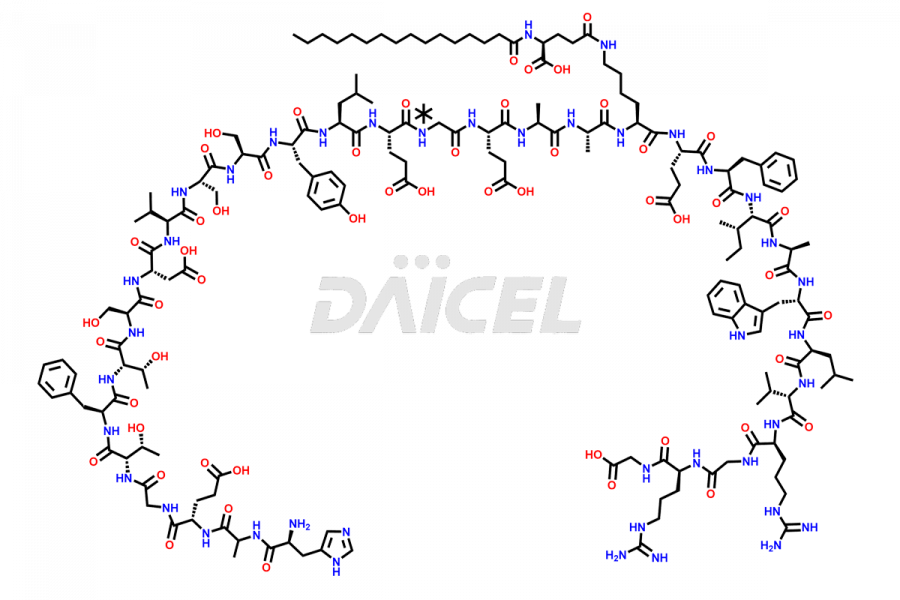
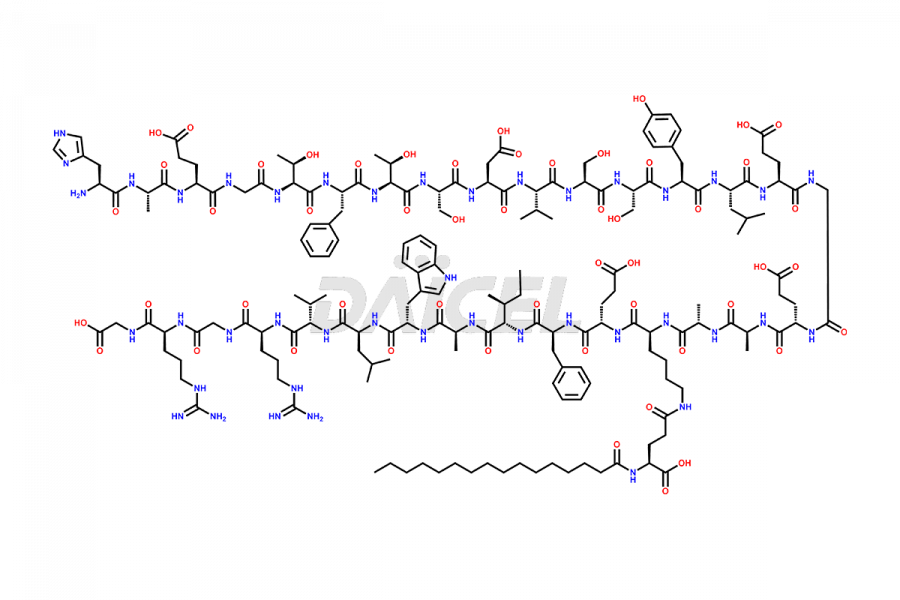
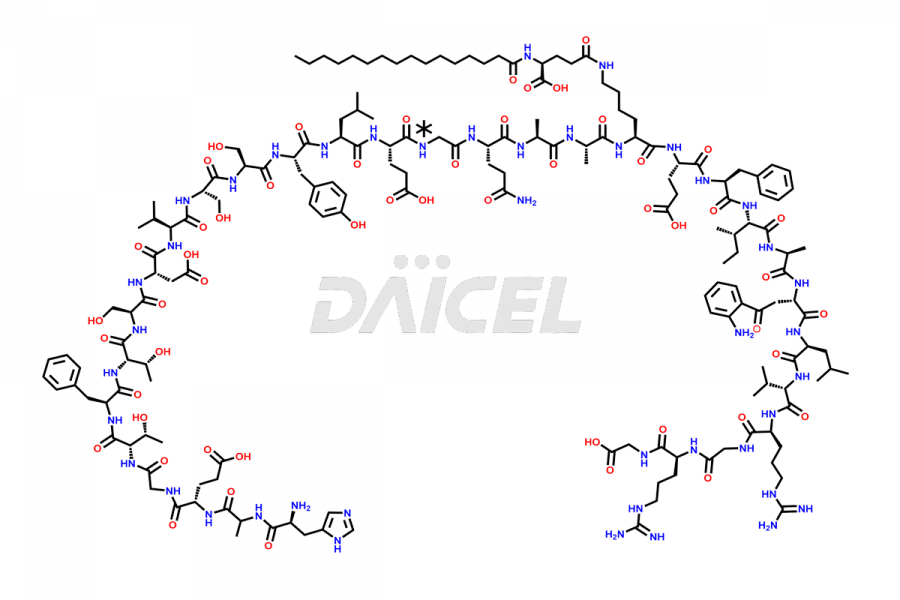
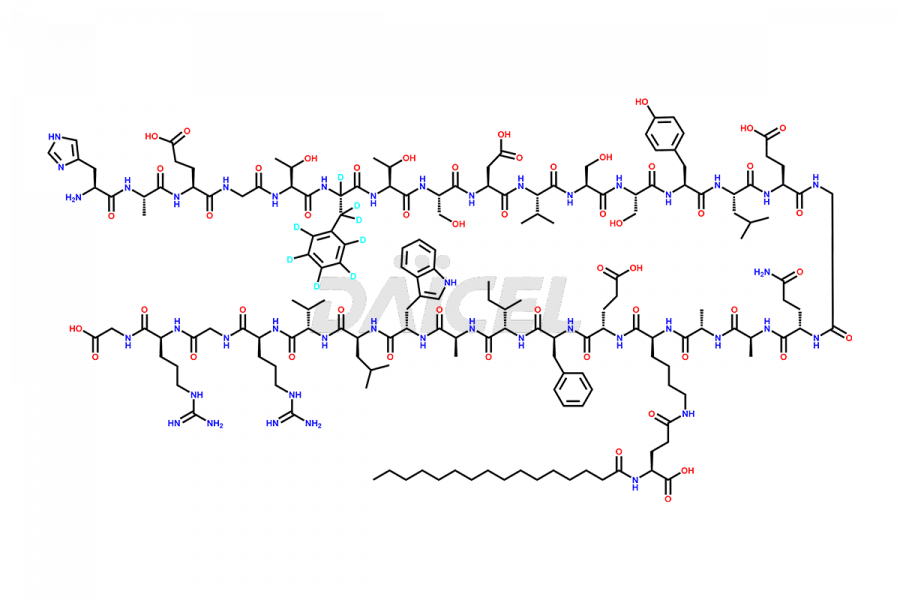
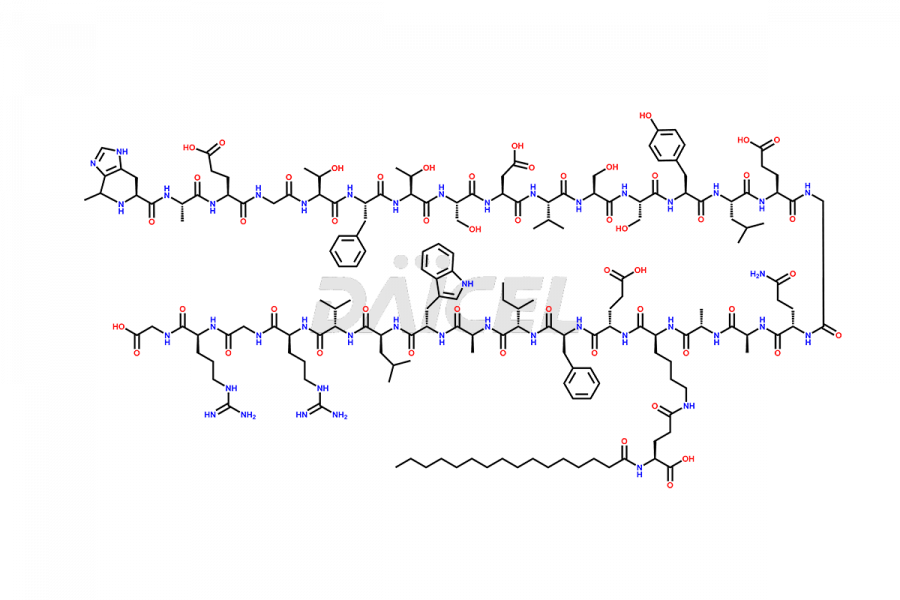
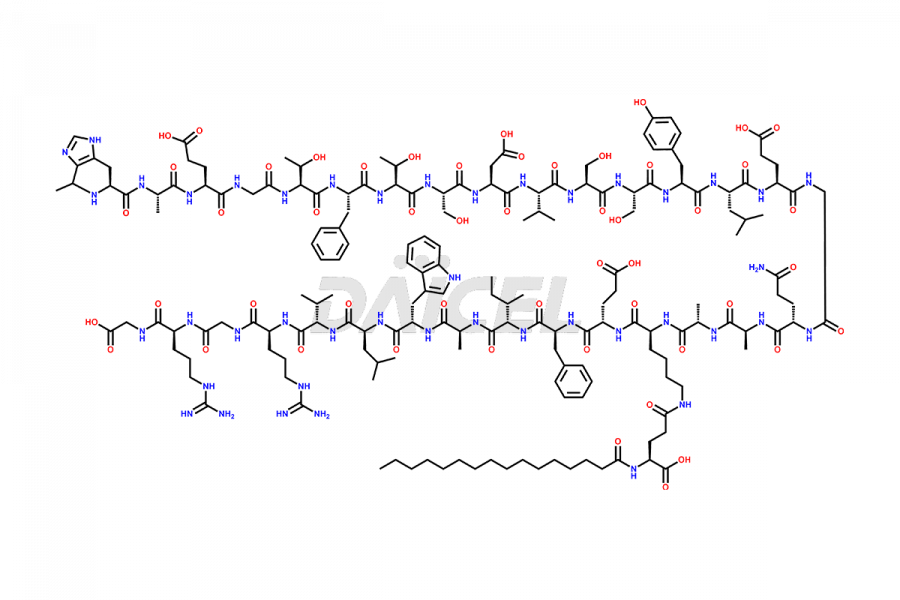
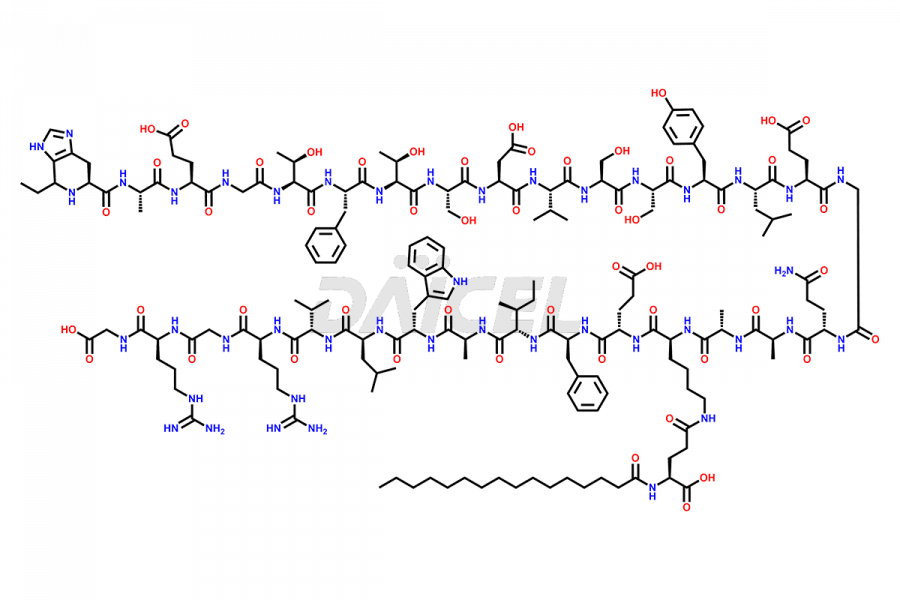
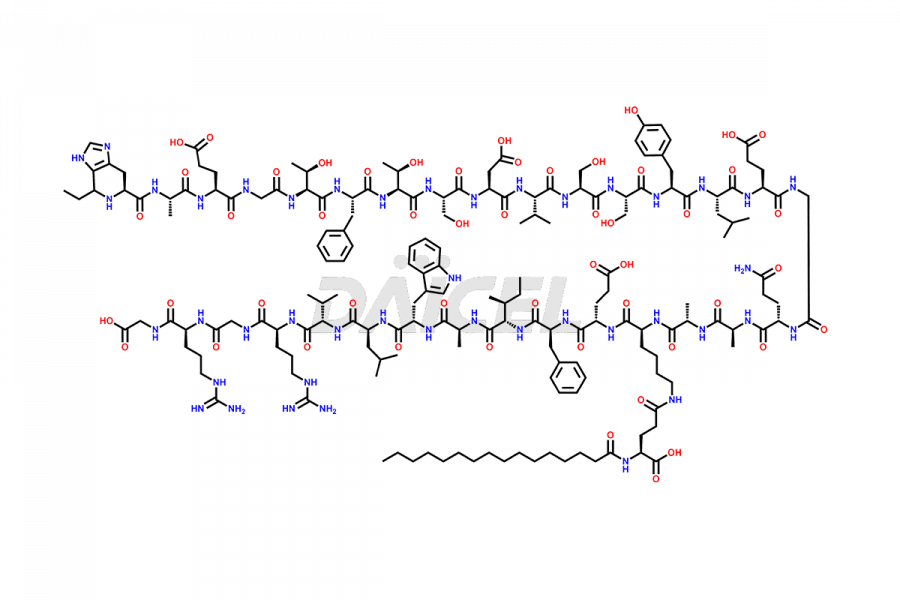
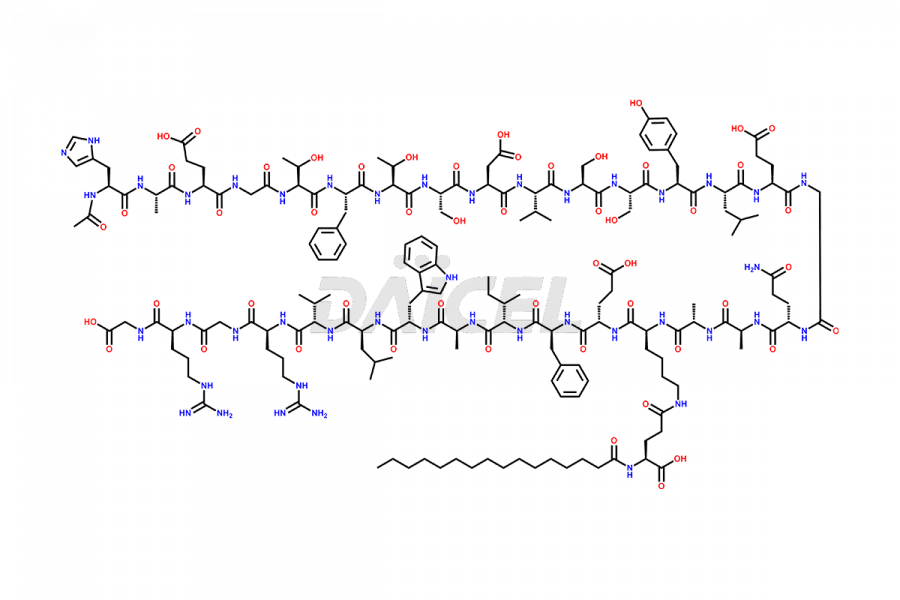
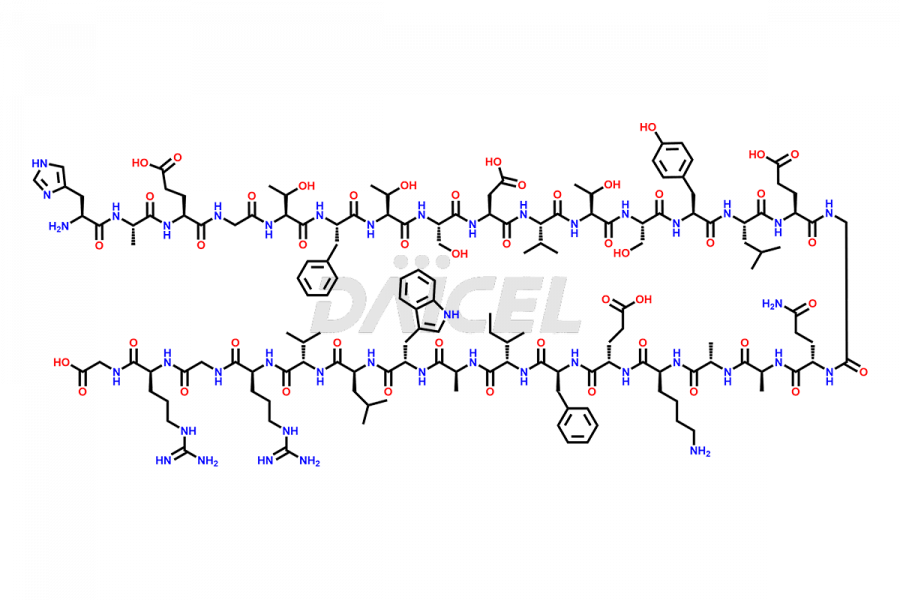
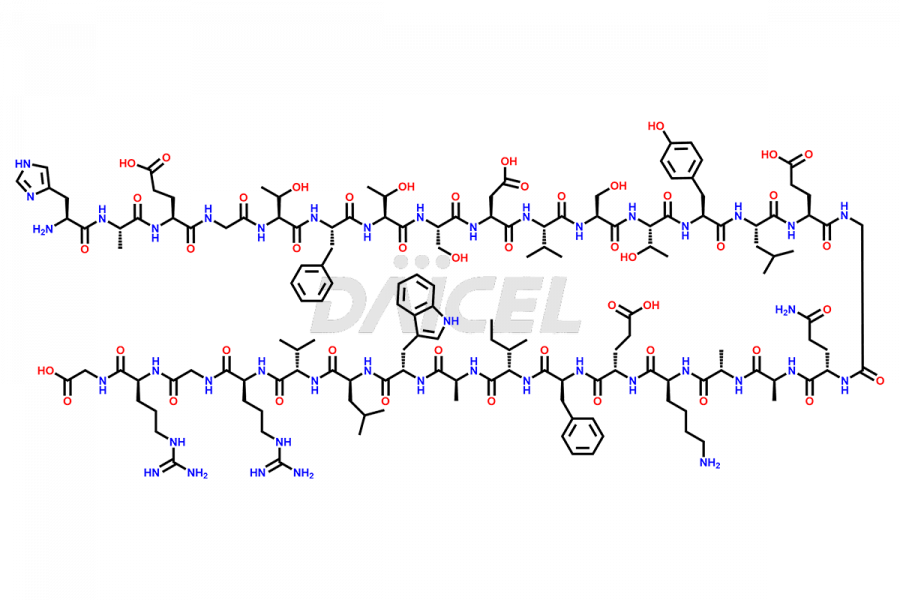
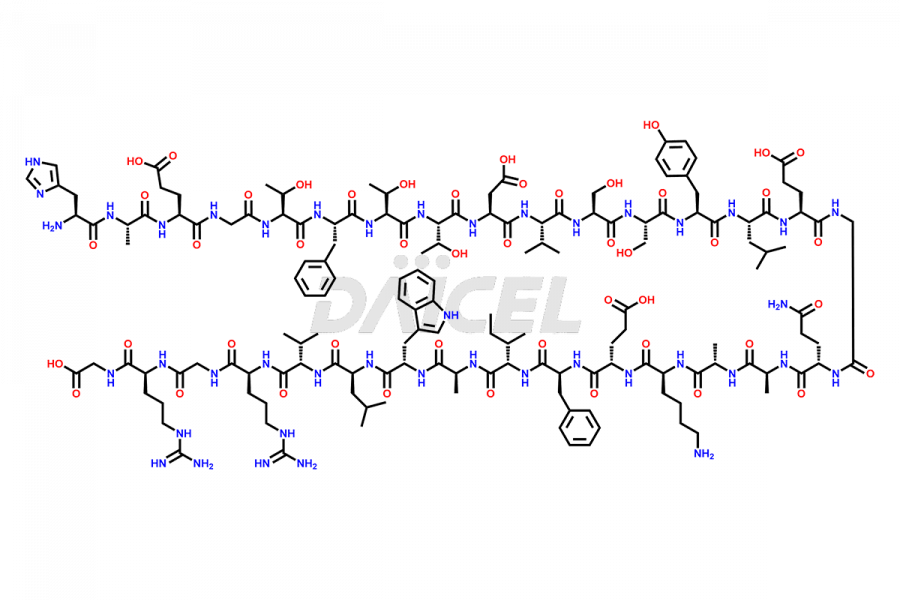
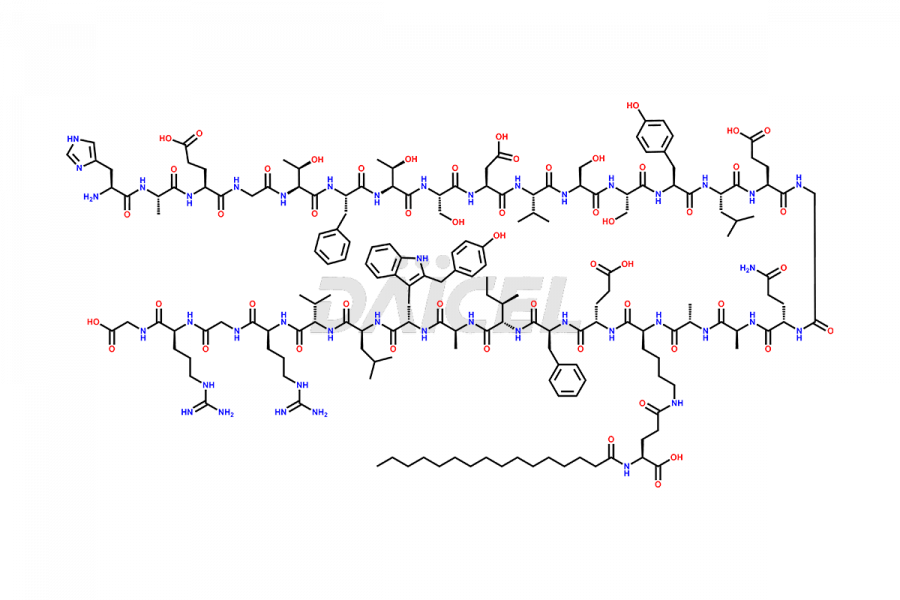
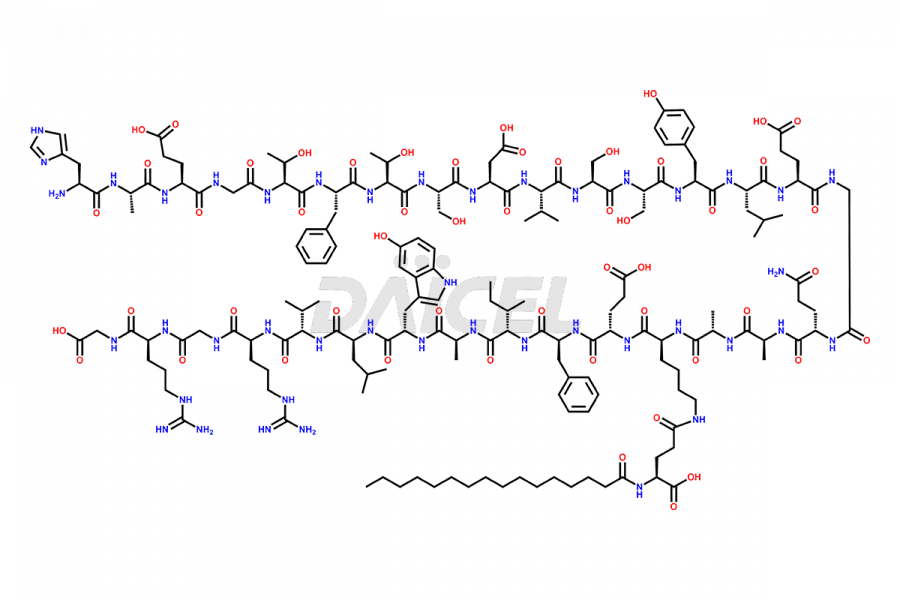
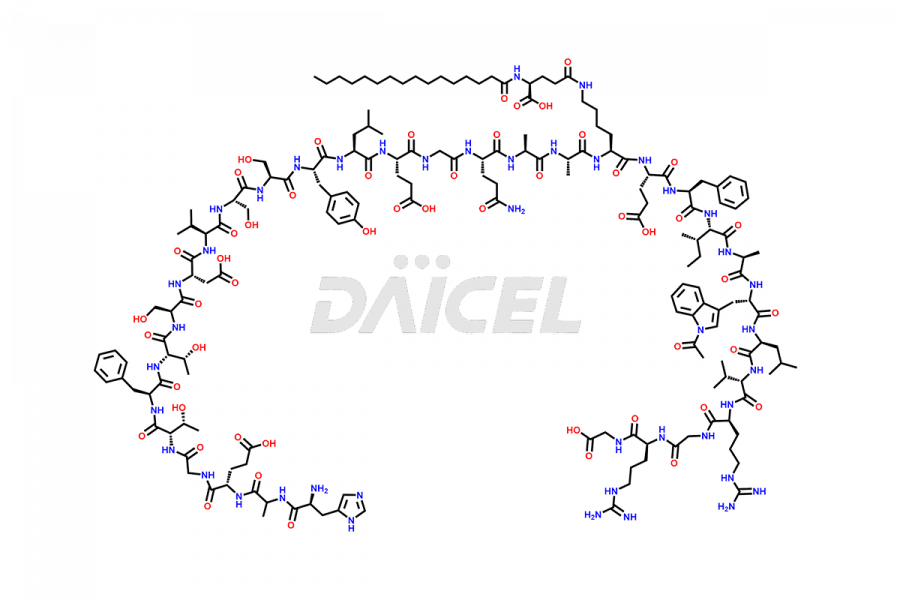
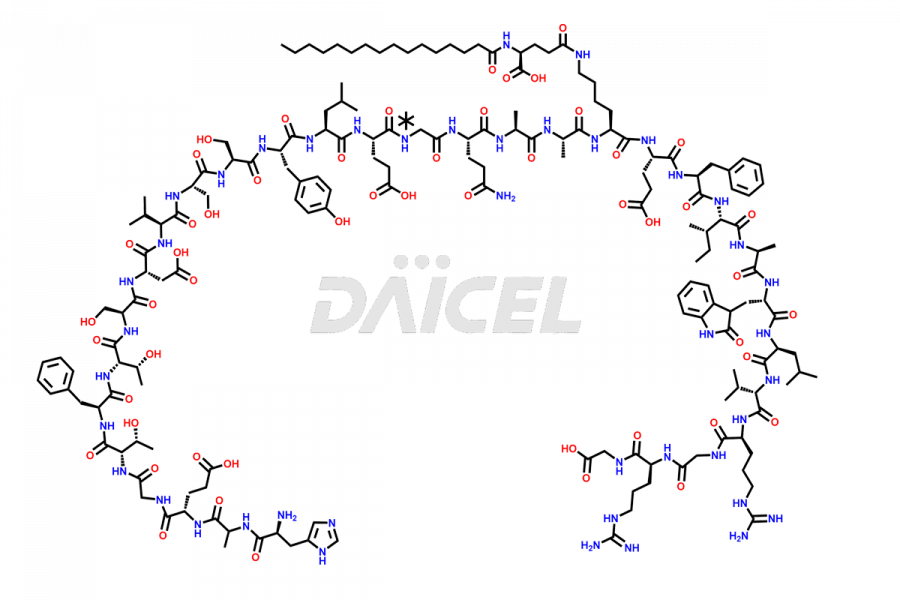
Liraglutide
General Information
Liraglutide Impurities and Liraglutide
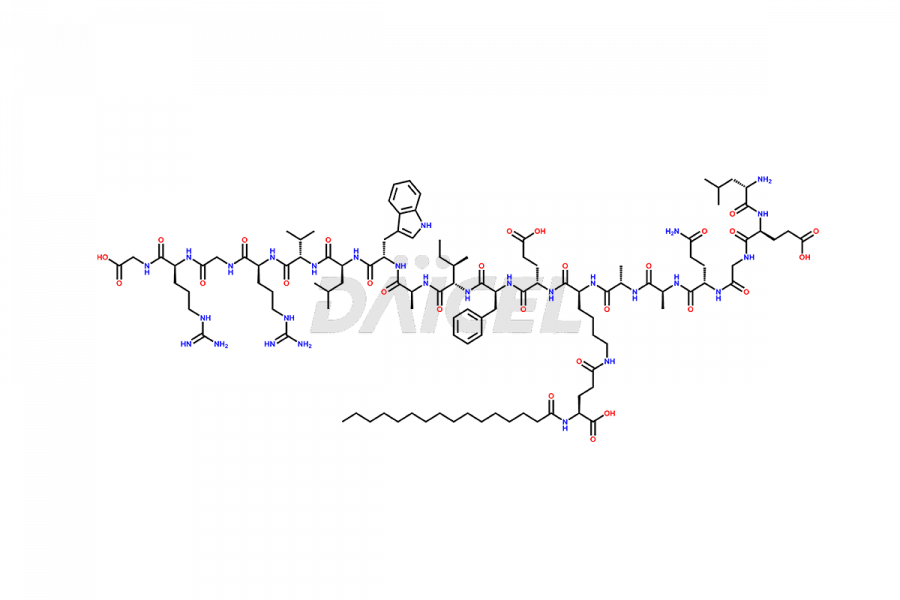
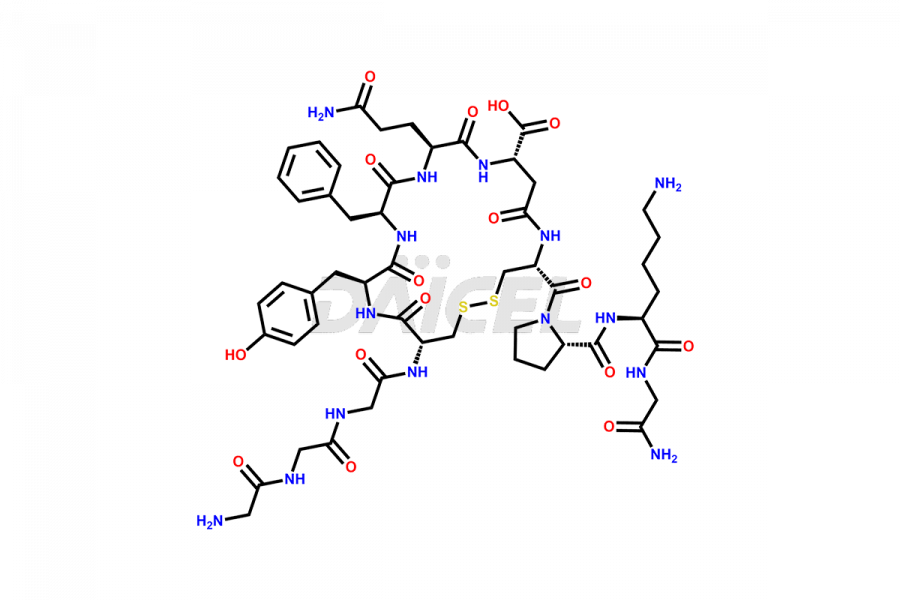
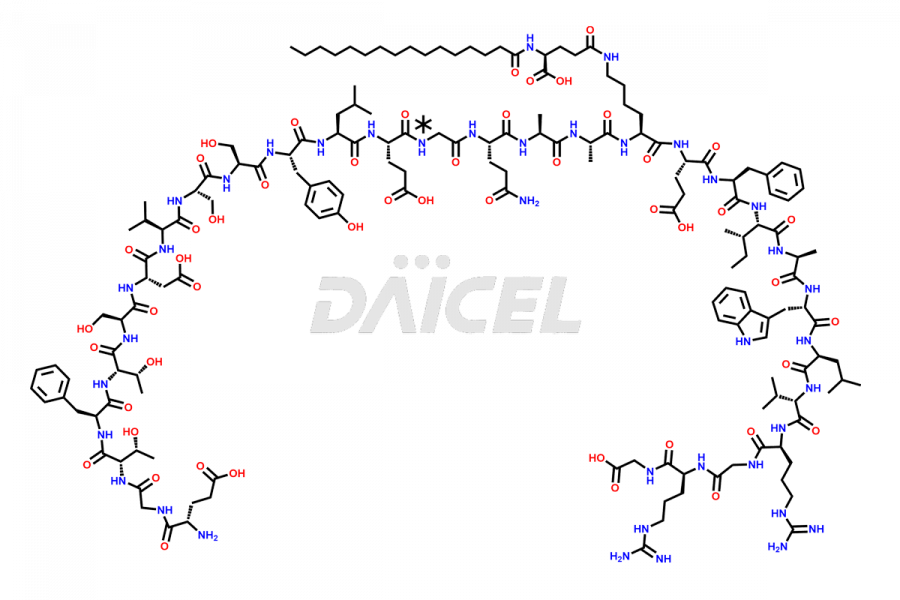
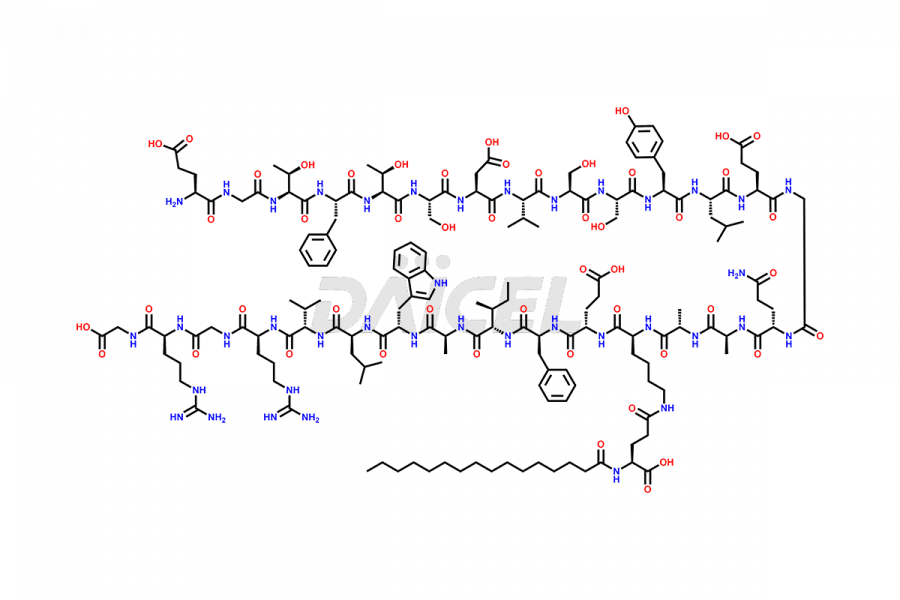
Daicel Pharma synthesizes high-quality Liraglutide impurities, Des-Gly (29)-Liraglutide, Des-Gly (4)-Liraglutide, Des-Ser (8)-Liraglutide, Glu (17)-Liraglutide, Kyn (25)-Liraglutide, and Trp(O)-Liraglutide, which helps in quality, stability, and biological safety analysis of the API of Liraglutide. We offer custom synthesis of Liraglutide impurities and can supply globally.
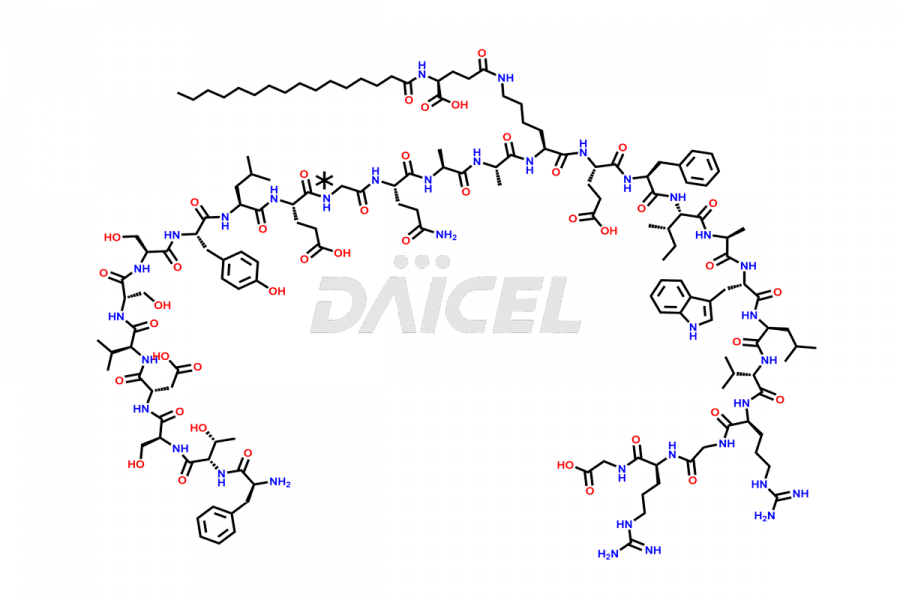
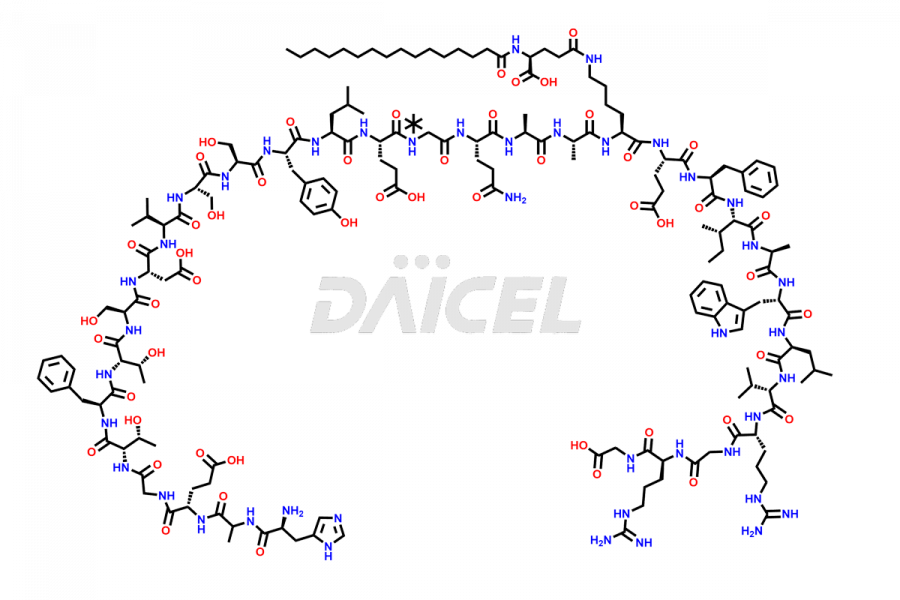
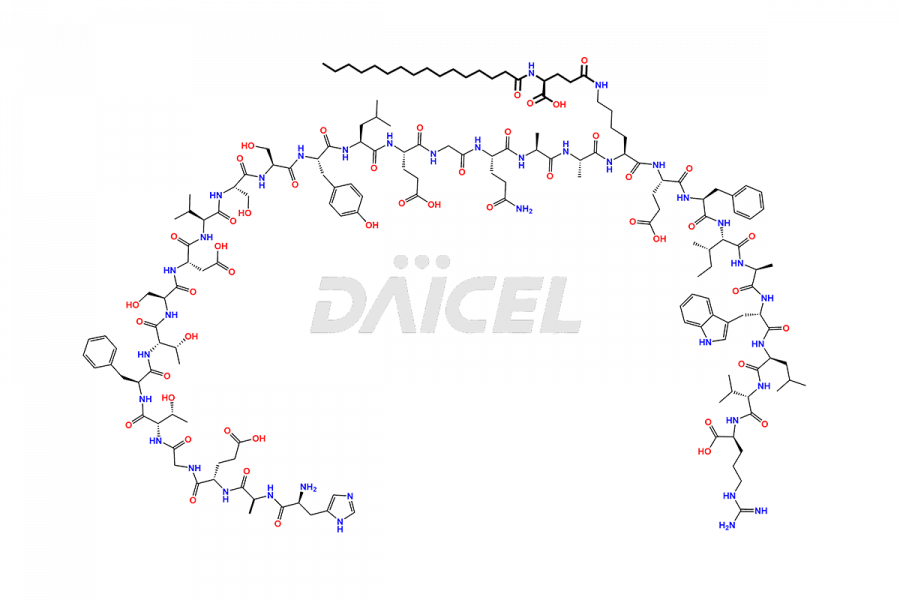
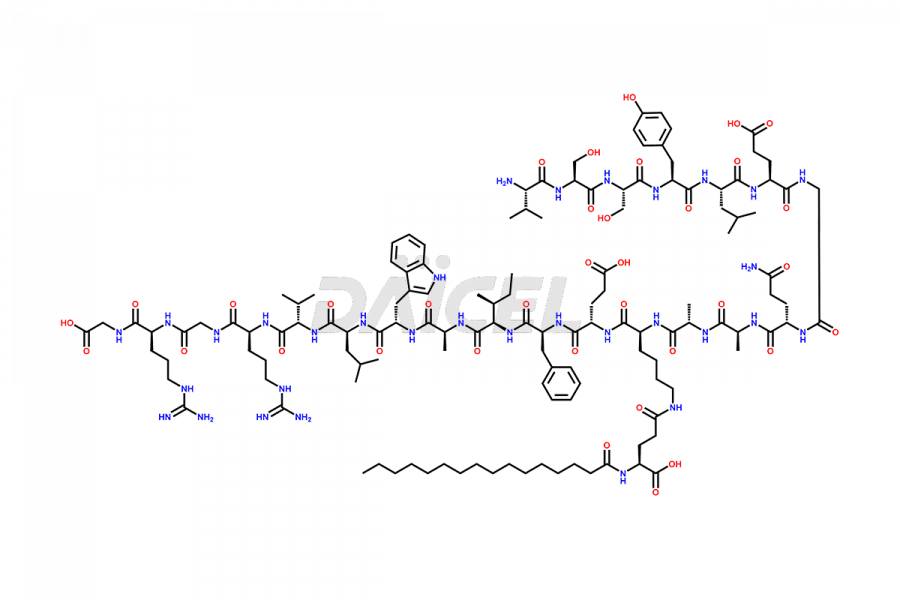
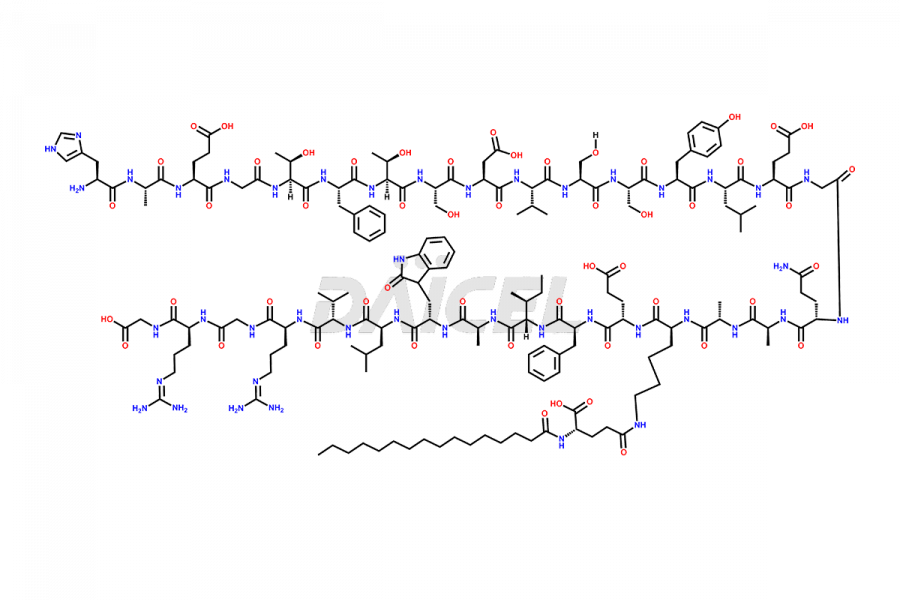
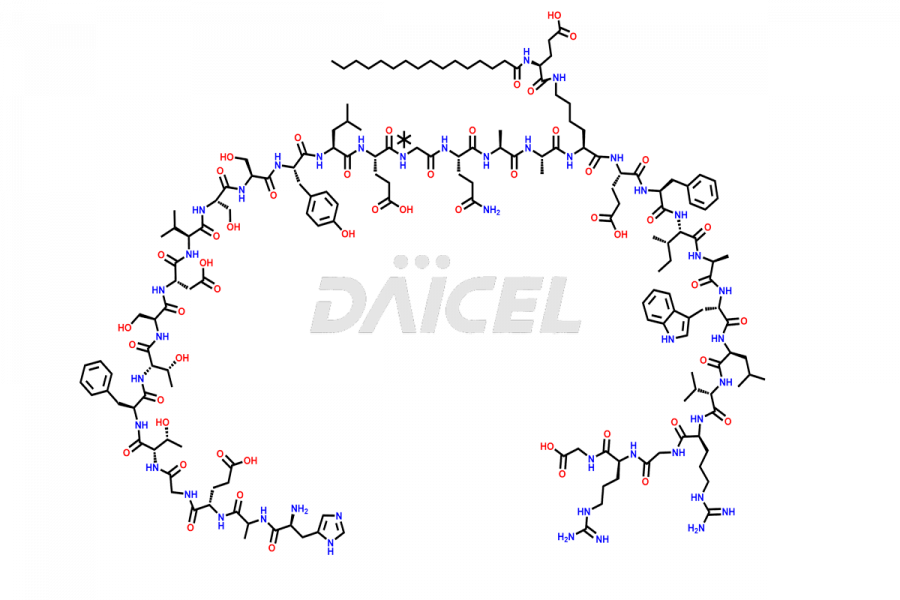
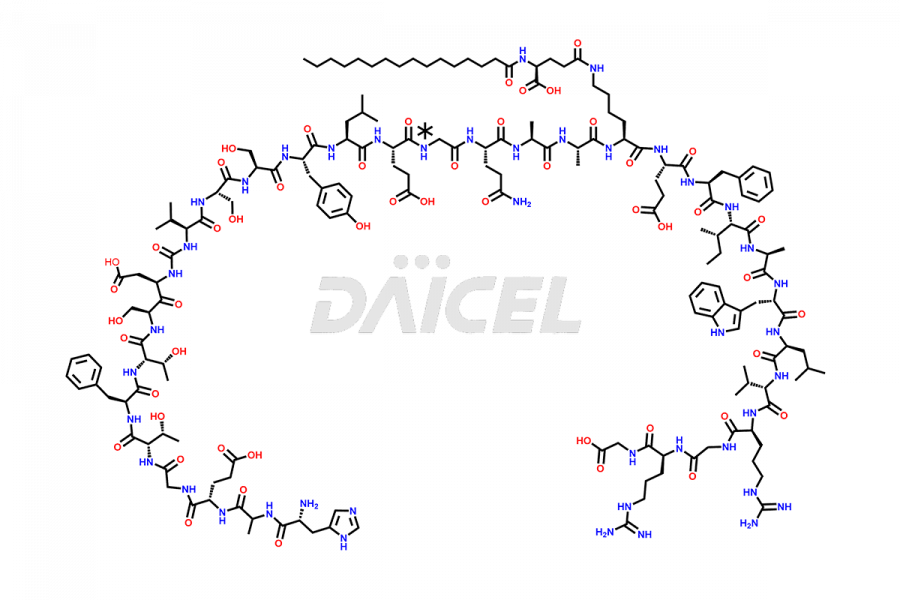
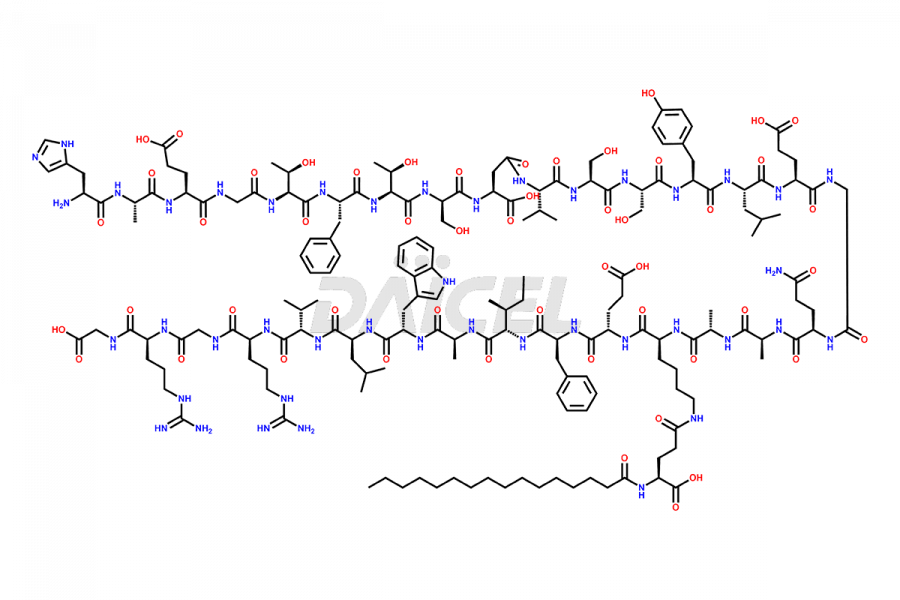
Liraglutide [CAS: 204656-20-2] is a medication categorized as a Glucagon-Like Peptide-1 (GLP-1) receptor agonist. It is commonly used to treat type 2 diabetes and prevent cardiovascular complications that may arise due to diabetes.
Liraglutide: Use and Commercial Availability
Liraglutide, available under the brand name Saxenda, is used to treat chronic weight management. It aids long-term weight management in adults with obesity (BMI≥30 kg/m2) or overweight (BMI≥27 kg/m2) and at least one weight-related comorbidity such as hypertension, dysglycaemia, etc. It is indicated as an adjunct to a reduced-calorie diet.
Victoza, another brand under which Liraglutide is available, improves glycemic control in patients aged 10 or older with type 2 diabetes mellitus. It also minimizes the risk of major adverse cardiovascular events in adult patients with type 2 diabetes and established cardiovascular disease. It is available as a solution in subcutaneous injection, a pre-filled multi-dose pen that delivers doses of Liraglutide to the patients1. 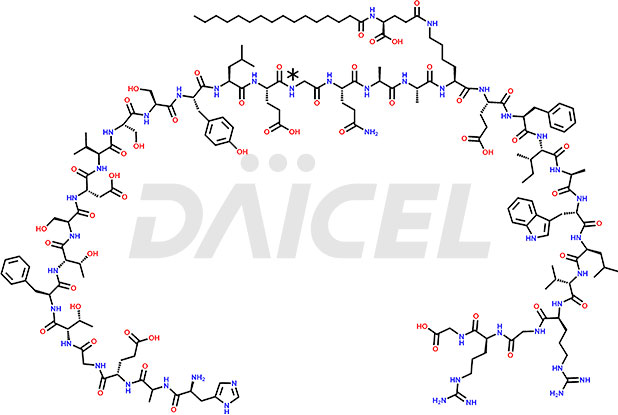

Liraglutide Structure and Mechanism of Action
The chemical formula of Liraglutide is C172H265N43O51 and its molecular weight is approximately 3751.2 g/mol.
Liraglutide is a synthetic, acylated human glucagon-like peptide-1 (GLP-1) receptor agonist. GLP-1 receptor is present in various areas of the brain involved in appetite regulation. It also inhibits the glucose-dependent release of glucagon and slows down gastric emptying, ultimately helping to regulate blood sugar levels more effectively.
Liraglutide Impurities and Synthesis
Liraglutide synthesis uses recombination technology in yeast and is chemically modified by the addition of the Glu-spaced hexadecanoic (palmitic) acid2 Liraglutide impurities include related substances, degradation products, residual solvents, inorganic impurities, and microbial impurities3. The manufacturers of Liraglutide perform rigorous analytical testing to detect and quantify these impurities to ensure the quality and safety of the drug.
At Daicel, we provide a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Liraglutide impurity standards, Des-Gly (29)-Liraglutide, Des-Gly (4)-Liraglutide, Des-Ser (8)-Liraglutide, Glu (17)-Liraglutide, Kyn (25)-Liraglutide, and Trp(O)-Liraglutide, with complete characterization data including 1H NMR, 13C NMR, IR, MS3, and HPLC purity. We also provide 13C-DEPT and CHN on request. Further, we provide a complete characterization report upon delivery. Daicel offers highly pure isotope-labeled standards of Liraglutide in bioanalytical research and BA/BE studies. Bioanalytical data is vital in the development of chemical and biological drugs with isotope data in CoA.
References
FAQ's
References
- Victoza-Product monograph-Novo Nordisk
- Liselotte Bjerre Knudsen, Per Olaf Huusfeldt, Per Franklin Nielsen, Niels C. Kaarsholm, Helle Birk Olsen, S0ren Erik Bj0rn, Freddy Zimmerdahl Pedersen, Kjeld Madsen; Novo Nordisk A/S, “Derivatives of GLP-1 analogs”, Denmark, US patent, US 6,268,343 Bl, July 31, 2001
- Shah, Mubarak Hasan; Reddy, Padmanabha Reddy Yeragam; Bhawanishankar, Madhira; Raja, Mokkaisamy Jegadeesh; Pillai, Manoj,” UHPLC-MS/MS determination of GLP-1 analogue, liraglutide a bioactive peptide in human plasma”, European Journal of Biomedical and Pharmaceutical Sciences, Volume: 4, Issue: 3, Pages: 304-311, 2017
Frequently Asked Questions
How are Liraglutide impurities identified?
Liraglutide impurities can be identified using analytical methods such as high-performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS), etc.
How are Liraglutide impurities purified after synthesis?
Liraglutide impurities can be purified after synthesis using various techniques such as preparative HPLC, flash chromatography, or crystallization. These methods involve separating the impurities from the main product based on their chemical properties and physical characteristics.
What are the temperature conditions required to store Liraglutide impurities?
Liraglutide impurities should be stored at recommended temperature conditions, typically 2-8°C or -20°C, depending on their stability. Improper storage can lead to degradation and loss of purity, so it's important to follow the manufacturer's instructions.
Why are Liraglutide impurities essential in drug development?
Liraglutide impurities can affect drug efficacy and safety. It is observed that impurities can cause adverse effects such as allergic reactions, injection site reactions, gastrointestinal disturbances, pancreatitis, or liver damage due to their toxicity or carcinogenic nature. Identifying and quantifying the impurities is essential during drug development.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.