Lisinopril
General Information
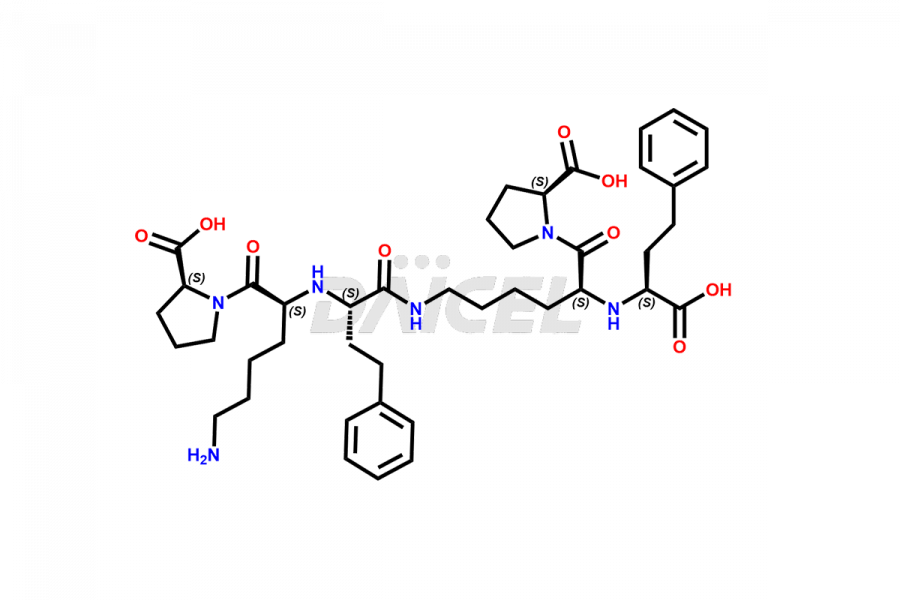
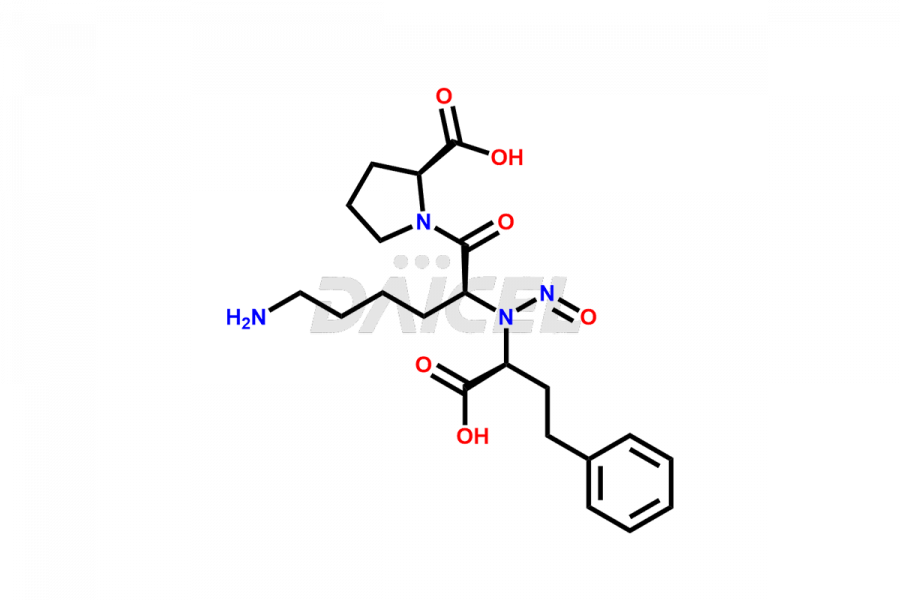
Lisinopril Impurities and Lisinopril
Daicel Pharma offers excellent-quality Lisinopril impurities, such as Lisinopril EP Impurity J, Lisinopril Impurity- G, and N-Nitroso Lisinopril. They are vital for evaluating Lisinopril quality, stability, and biological safety. Further, Daicel Pharma specializes in the custom synthesis of Lisinopril impurities and ensures their worldwide delivery.
Levonorgestre [CAS: 76547-98-3] is a synthetic peptide derivative and a vasoconstrictor. As an antihypertensive drug, it can dilate vascular blood vessels. It prevents the conversion of angiotensin I to angiotensin II, which constricts coronary blood vessels.
Lisinopril: Use and Commercial Availability
Prinivil, Qbrelis, and Zestril are brands of Lisinopril, available as oral formulations. Lisinopril is approved by the US FDA. It treats patients with hypertension and myocardial infarction. It combines with other antihypertensive drugs to treat heart failure. Further, Lisinopril helps to lower the side effects caused by anti-cancer medicines.
Lisinopril Structure and Mechanism of Action
The chemical name of Lisinopril is N2-[(1S)-1-Carboxy-3-phenylpropyl]-L-lysyl-L-proline. The chemical formula for Lisinopril is C21H31N3O5, and its molecular weight is approximately 405.49 g/mol.
Lisinopril suppresses the renin-angiotensin-aldosterone system. It blocks the angiotensin-converting enzyme (ACE), causing decreased vasopressor activity and aldosterone secretion.
Lisinopril Impurities and Synthesis
During Lisinopril synthesis1, impurities form that can affect drug safety and efficacy. They form during the synthetic process, purification, or storage of Lisinopril. And so, Lisinopril impurities must be controlled and monitored throughout the drug’s development process.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Lisinopril impurities, which includes Lisinopril EP Impurity J, Lisinopril Impurity- G, and N-Nitroso Lisinopril. We provide the CoA from a cGMP-compliant analytical facility. It gives complete characterization data2 such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We provide additional analytical data on request. Daicel Pharma can prepare any unidentified Lisinopril impurity or degradation product. Moreover, Daicel Pharma offers a highly purified, stable isotope-labeled standard of Lisinopril. We provide a complete characterization report to our clients upon delivery.
References
FAQ's
References
- Patchett, Arthur A.; Harris, Elbert E.; Wyvratt, Matthew J.; Tristram, Edward W., Carboxyalkyl dipeptide derivatives, process for preparing them and pharmaceutical composition containing them, EP12401B1, Dec 10, 1979, Merck and Co., Inc., United States (https://www.lens.org/lens/search/patent/list?q=EP0012401)
- Qin, Xue Zhi; Nguyen, Diem Suong T.; Ip, Dominic P., Separation of lisinopril and its RSS diastereoisomer by micellar electrokinetic chromatography, Journal of Liquid Chromatography, Volume: 16, Issue: 17, Pages: 3713-34, 1993, DOI: (10.1080/10826079308019663)
Frequently Asked Questions
Which analytical method detects and analyzes Lisinopril impurities?
High-performance liquid chromatography coupled with mass spectrometry (HPLC/MS) detects and analyzes Lisinopril impurities.
How do Lisinopril impurities form in the drug substance?
Unreacted intermediates, by-products, reagents, and reaction conditions result in the formation of Lisinopril impurities.
What causes the degradation of Lisinopril in the drug product?
Exposure to light is the root cause of the degradation of Lisinopril.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.