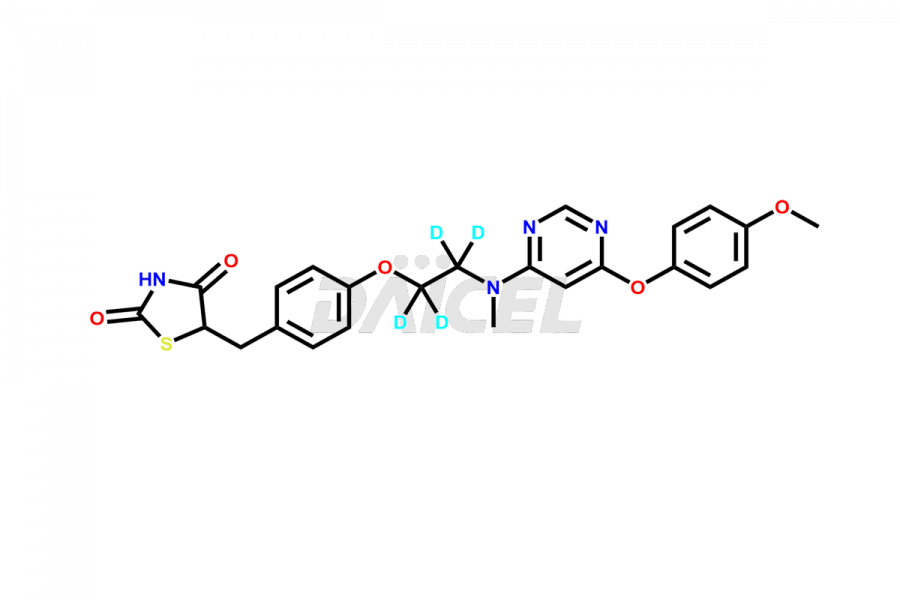
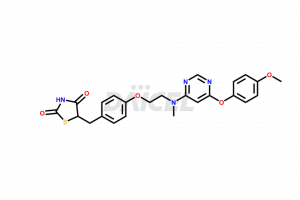
Lobeglitazone
General Information
Lobeglitazone Impurities and Lobeglitazone
Daicel Pharma offers the best-quality Lobeglitazone impurities and labeled standards. They are vital for evaluating Lobeglitazone quality, stability, and biological safety. Furthermore, Daicel Pharma specializes in the custom synthesis of Lobeglitazone impurities and ensures their worldwide delivery.
Lobeglitazone [CAS: 607723-33-1] is a thiazolidinedione derivative. It is an antidiabetic drug and an insulin sensitizer. Lobeglitazone was developed by Chong Kun Dang Pharmaceutical in South Korea, where it got initial approval. It reduces insulin resistance and improves blood sugar levels in diabetic patients.
Lobeglitazone: Use and Commercial Availability
Lobeglitazone treats type 2 diabetes. It reduces blood sugar levels and improves lipid and liver profiles. It is effective in the treatment of Non-alcoholic Fatty Liver Disease. In addition, it treats diabetic patients with ischemic stroke. It also reduces the risk of cardiovascular events in patients. It is available as an oral formulation under the brand Duvie.
Lobeglitazone Structure and Mechanism of Action
The chemical name of Lobeglitazone is 5-[[4-[2-[[6-(4-Methoxyphenoxy)-4-pyrimidinyl]methylamino]ethoxy]phenyl]methyl]-2,4-thiazolidinedione. The chemical formula for Lobeglitazone is C24H24N4O5S, and its molecular weight is approximately 480.54 g/mol.
Lobeglitazone binds insulin by activating Peroxisome Proliferator-Activated Receptor gamma (PPAR-γ) within fat cells. It also lowers hemoglobin A1C (HbA1C) levels.
Lobeglitazone Impurities and Synthesis
During Lobeglitazone synthesis1, impurities form that can affect drug safety and efficacy. They form during manufacturing, purification, or storage of Lobeglitazone. Therefore, Lobeglitazone impurities must be controlled and monitored throughout the drug’s development.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Lobeglitazone impurities and labeled standards. We provide the CoA from a cGMP-compliant analytical facility. It gives complete characterization data2 such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We provide additional analytical data on request. Daicel Pharma can prepare any unidentified Lobeglitazone impurity or degradation product. In addition, Daicel Pharma offers a highly purified, stable deuterium-labeled standard, Lobeglitazone D4. We provide clients with a complete characterization report upon delivery.
References
- Hong, Chung-Il; Ahn, Soon-Kil; Kim, Bok-Young; Ahn, Joong-Bok; Lee, Do-Young; Lee, Hong-Woo; Shin, Jae-Soo, Thiazolidinedione derivatives and pharmaceutical composition comprising the same, WO03080605A1, Mar 28, 2002, Chong Kun Dang Pharmaceutical Corp. Republic of Korea. (https://patents.google.com/patent/WO2003080605A1/und)
- Kim, Bora; Shin, Hyun-Suk; Kim, Jung-Ryul; Lim, Kyung-Soo; Yoon, Seo Hyun; Yu, Kyung-Sang; Shin, Sang-Goo; Jang, In-Jin; Cho, Joo-Youn, Quantitative and Qualitative Analysis of CKD-501, Lobeglitazone, in Human Plasma and Urine Using LC-MS/MS and Its Application to a Pharmacokinetic Study, Chromatographia, Volume: 75, Issue: 11-12, Pages: 671-677, 2012 DOI: (10.1007/s10337-012-2238-0)
Frequently Asked Questions
Under what conditions does Lobeglitazone degrade?
Lobeglitazone degrades on exposure to light, moisture, and oxidation conditions.
Which analytical method can identify and separate the degradation products of Lobeglitazone present in the drug?
Liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) can identify and separate the degradation products of Lobeglitazone.
Why is it essential to remove Lobeglitazone impurities from the drug?
Lobeglitazone impurities can affect the drug's quality, safety, and efficacy. Hence, it is essential to remove them from the drug.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.