Lofexidine
General Information
Lofexidine Impurities and Lofexidine
Daicel Pharma offers superior-quality Lofexidine impurities and labeled standards. It is vital for evaluating Lofexidine quality, stability, and biological safety. Further, Daicel Pharma excels in the custom synthesis of Lofexidine impurities and ensures their global delivery.
Lofexidine [CAS: 31036-80-3] is an imidazole derivative and an antihypertensive agent. It is a non-opioid adrenergic receptor agonist. It is effective in managing opioid withdrawal symptoms with abrupt stoppage of opioids. In the past, Lofexidine was part of the treatment for patients with high blood pressure. Now, it is a part of the treatment of opiate addiction.
Lofexidine: Use and Commercial Availability
Lofexidine is available as an oral formulation under Lucemyra. It helps in reducing opioid withdrawal symptoms in patients who have abruptly discontinued opioids. The opioid withdrawal symptoms include nausea, abdominal pain, diarrhea, tension, etc. It also treats patients with chronic hypertension. Further, it treats patients with narcotic addiction.
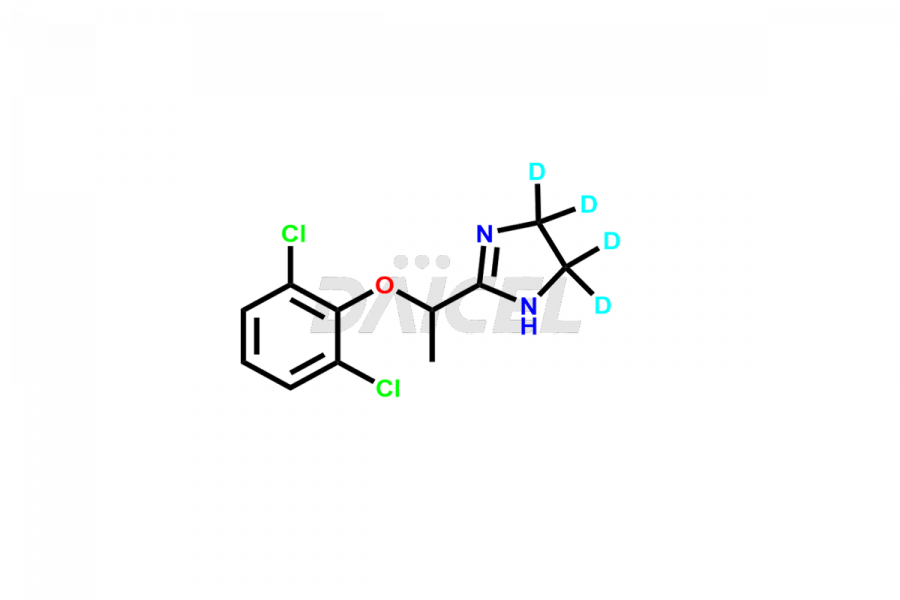
Lofexidine Structure and Mechanism of Action
The chemical name of Lofexidine is 2-[1-(2,6-Dichlorophenoxy)ethyl]-4,5-dihydro-1H-imidazole. The chemical formula for Lofexidine is C11H12cl2N2O, and its molecular weight is approximately 259.13 g/mol.
Lofexidine binds to adrenergic receptors and decreases norepinephrine release. It stops cAMP synthesis, causing neural firing suppression.
Lofexidine Impurities and Synthesis
When synthesizing Lofexidine1, the formation of impurities may affect drug safety and efficacy. They form during the synthetic process, storage, or purification of Lofexidine. Lofexidine impurities need constant control and monitoring to ensure that the drug is safe, effective, and stable.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Lofexidine impurities and labeled standards. The CoA is from a cGMP-compliant analytical facility and consists of the complete characterization data2, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We give additional spectral data on request. Daicel Pharma can prepare any unidentified Lofexidine impurity or degradation product. In addition, Daicel Pharma offers a highly purified, stable isotope-labeled standard, Lofexidine D4. Clients receive a complete characterization report upon delivery.
References
- Baganz, Horst; May, Hans J., Imidazoline derivatives and processes for the production thereof, US3966757A, Apr 8, 1975, Nordmark-Werke G.m.b.H. (https://www.lens.org/lens/search/patent/list?q=US3966757)
- Mastanamma, Sk; Satya, Anjali T.; Tejaswi, J.; Chiranmai, M.; Hema, Latha K., Separation and identification of forced degradation products of lofexidine by using LC-MS/MS, Asian Journal of Pharmaceutical and Clinical Research, Volume: 15, Issue: 9, Pages: 210-222, 2022 DOI: (10.22159/ajpcr.2022v15i9.45117)
Frequently Asked Questions
Under what conditions does Lofexidine degrade in the drug?
Lofexidine degrades under acid, alkaline hydrolysis, and oxidative conditions in the drug.
How do nitrosamine impurities form in the Lofexidine drug?
Amines and nitrates are the root cause of forming nitrosamine impurities in Lofexidine.
Why is it necessary to remove Lofexidine impurities from the drug?
Lofexidine impurities can affect drug safety and quality and pose health risks, so it is necessary to remove them from the drug.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.