Loperamide
General Information
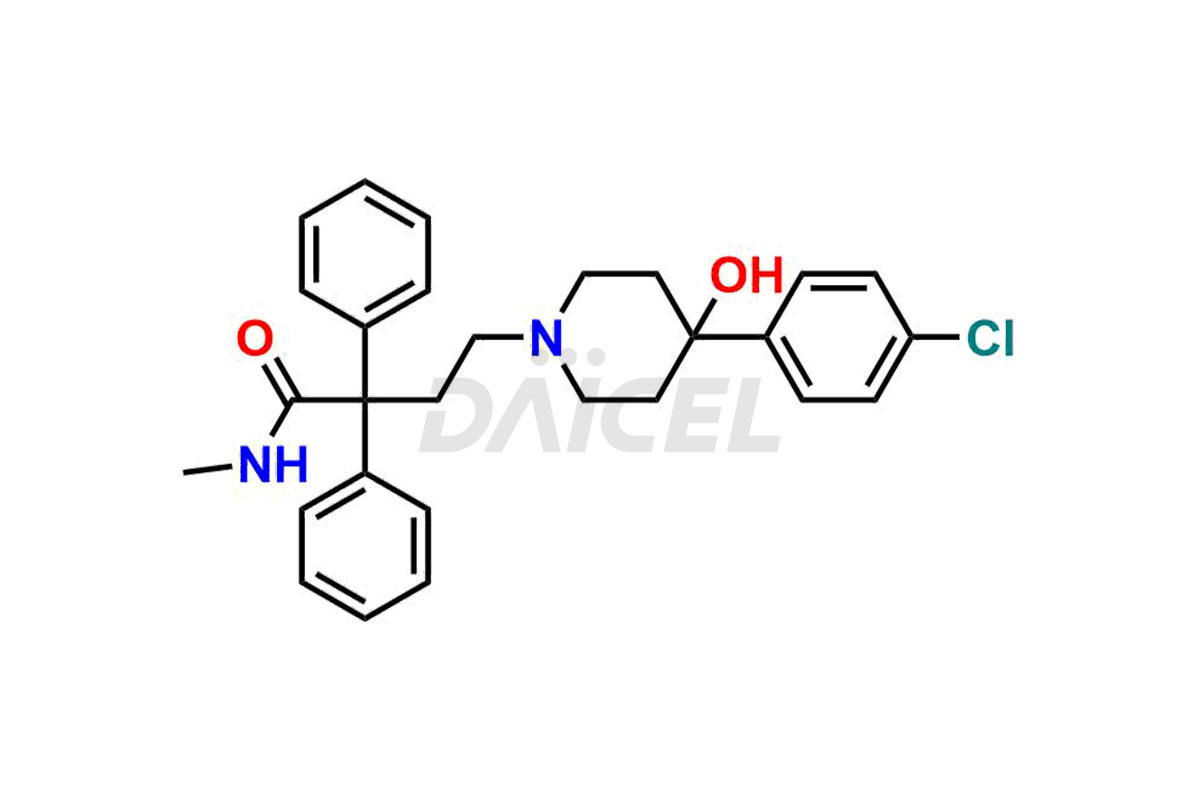
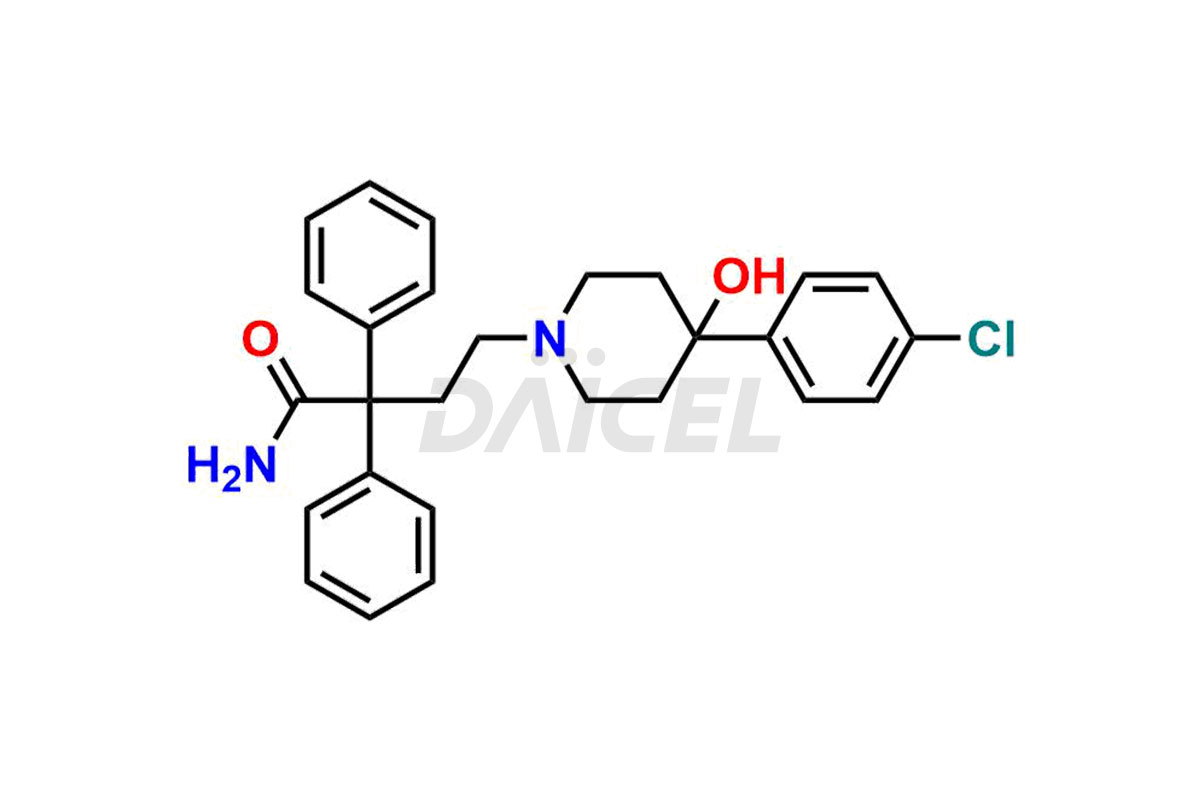
Loperamide Impurities and Loperamide
For evaluating the purity and safety of Loperamide, an active pharmaceutical ingredient, Daicel Pharma offers a customized synthesis of Loperamide impurity standards. These impurity standards include crucial compounds such as Desmethyl Loperamide and Didesmethyl Loperamide. Additionally, Daicel Pharma provides worldwide delivery options for Loperamide impurity standards.
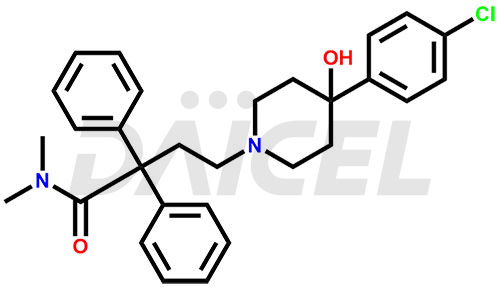
Loperamide [CAS: 53179-11-6] is a long-acting synthetic antidiarrheal agent. It primarily acts on opiate receptors in the intestine to effectively treat diarrhea. Loperamide is not substantially absorbed from the gut and does not affect the central nervous system.
Loperamide: Use and Commercial Availability
Imodium and Imodium A-D are the brand names for the drug Loperamide, an over-the-counter oral antidiarrheal agent. Initially categorized as a Schedule V drug by the FDA due to its potential for opioid-like abuse, it is now FDA-approved for treating various forms of diarrhea. It is also commonly used off-label to manage chemotherapy-induced diarrhea. In recent years, there has been increased non-medical use of Loperamide for purposes such as self-management of opioid withdrawal symptoms and recreational use. It relieves diarrhea, including Travelers’ Diarrhea, and alleviates chemotherapy-related diarrhea.
Loperamide Structure and Mechanism of Action
The chemical name of Loperamide is 4-(4-Chlorophenyl)-4-hydroxy-N, N-dimethyl-α, α-diphenyl-1-piperidinebutanamide. Its chemical formula is C29H33ClN2O2, and its molecular weight is approximately 477.0 g/mol.
Loperamide slows intestinal motility by inhibiting the release of acetylcholine and prostaglandins.
Loperamide Impurities and Synthesis
Impurities in Loperamide refer to unintended substances present in the drug formulation, distinct from the desired active pharmaceutical ingredient (API). They can originate from various sources, including manufacturing, starting materials, or interactions with other compounds. Common Loperamide impurities include related compounds, degradation products, residual solvents, and process-related impurities. Strict monitoring and control of impurity levels during manufacturing ensure the safety, efficacy, and quality of Loperamide. Regulatory guidelines and limits govern the acceptable levels of impurities in the final drug product, aiming to minimize potential adverse effects and maintain product purity.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for preparing Loperamide impurity standards, which include Desmethyl Loperamide and Didesmethyl Loperamide. Our Loperamide impurity standards have a detailed Certificate of Analysis (CoA) that provides a comprehensive characterization report. This report has data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis1, 2. Upon request, we give additional data like 13C-DEPT. Moreover, we can synthesize unknown Loperamide impurity standards and degradation products. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Leung, C. P.; Au-Yeung, C. Y., High-performance liquid chromatographic determination of loperamide hydrochloride in pharmaceutical preparations, Journal of Chromatography Volume: 449, Issue: 1, Pages: 341-4, 1988
- Leis, Hans Joerg, Gas chromatography/negative-ion chemical ionization mass spectrometry of tertiary amines following Hofmann degradation. Application to the characterization of Loperamide, Rapid Communications in Mass Spectrometry, Volume: 3 Issue: 10, Pages: 342-3, 1989
Frequently Asked Questions
How often are impurity levels in Loperamide tested and monitored?
Impurity levels in Loperamide are routinely tested and monitored throughout the manufacturing process. Regular testing is performed at different stages, including raw materials, in-process samples, and the final product, to ensure compliance with regulatory requirements and maintain product quality.
Can Loperamide impurities affect its stability under specific storage conditions?
Some impurities in Loperamide can contribute to its degradation and affect its stability under specific storage conditions. Proper storage and drug handling are essential to minimize its impact on stability.
What solvent is typically used for analyzing Loperamide impurities?
Methanol is a solvent used when analyzing many impurities in Loperamide.
What is the recommended storage temperature for Loperamide impurities?
Loperamide impurities should be stored, at a controlled room temperature, usually between 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.