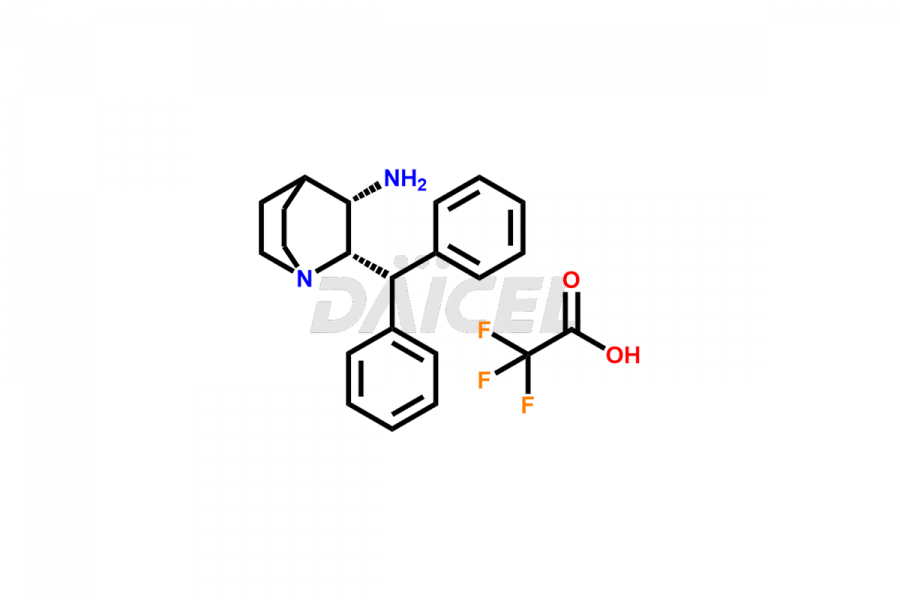
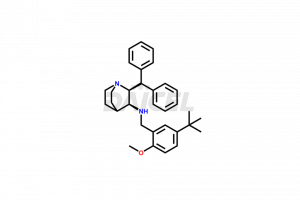
Maropitant
General Information
Maropitant Impurities and Maropitant
Daicel Pharma offers superior-quality Maropitant impurities, such as Maropitant (MRP) Diastereomer Impurity, Maropitant Enantiomer Impurity, and MRP-6 IMPURITY (TFA salt). It is vital for evaluating Maropitant quality, stability, and biological safety. Further, Daicel Pharma excels in the custom synthesis of Maropitant impurities and ensures their global delivery.
Maropitant [CAS: 147116-67-4] is a quinuclidine compound used in veterinary treatment. Developed by Zoetis1, it is a US FDA-approved veterinary anti-emetic. It is a selective neurokinin-1 (NK-1) receptor antagonist treating vomiting in dogs and cats.
Maropitant: Use and Commercial Availability
Maropitant is an anti-emetic for dogs and cats. It prevents nausea in dogs undergoing chemotherapy. It further treats vomiting in dogs caused by motion sickness. It is available as oral and injectable formulations under the brand Cerenia for dogs and cats in the US. Prevomax is the brand under which Maropitant is available as an injectable. In addition, it treats pain and is a stress response modulator during handling and hospitalization. Many generic formulations of Maropitant are available. It prevents peri-operative
Maropitant Structure and Mechanism of Action
The chemical name of Maropitant is (2S,3S)-N-[[5-(1,1-Dimethylethyl)-2-methoxyphenyl]methyl]-2-(diphenylmethyl)-1-azabicyclo[2.2.2]octan-3-amine. The chemical formula for Maropitant is C32H40N2O, and its molecular weight is approximately 468.67 g/mol.
Maropitant blocks neurokinin 1, substance P, a neurotransmitter in the central nervous system that causes vomiting in dogs.
Maropitant Impurities and Synthesis
When synthesizing Maropitant2, the formation of impurities may affect drug safety and efficacy. They form during the synthetic process, storage, or purification of Maropitant. Maropitant impurities need to have continuous control and monitoring to ensure that the drug is safe, effective, and stable.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Maropitant impurities, which includes Maropitant (MRP) Diastereomer Impurity, Maropitant Enantiomer Impurity, and MRP-6 IMPURITY (TFA salt). The CoA is from a cGMP-compliant analytical facility and consists of the complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We give additional spectral data on request. Daicel Pharma can prepare any unidentified Maropitant impurity or degradation product. In addition, Daicel Pharma offers highly purified, stable isotope-labeled standards of Maropitant. Clients can expect a complete characterization report upon delivery.
References
FAQ's
References
- https://www.zoetisus.com/products/petcare/cerenia
- Ito, Fumitaka; Kondo, Hiroshi; Shimada, Kaoru; Nakane, Masami; Lowe, John Adams, III; Rosen, Terry Jay; Yang, Bingwei Vera, Quinuclidine derivatives, WO9221677A1, Apr 28, 1992, Pfizer Inc., United States (https://www.lens.org/lens/search/patent/list?q=WO9221677)
Frequently Asked Questions
How do Maropitant impurities form in the drug?
Maropitant impurities form during synthesis and from unreacted starting materials, by-products, reagents, and solvents during manufacturing.
Why is it necessary to remove Maropitant impurities from the drug?
Maropitant impurities can affect drug safety and quality and pose health risks, so it is necessary to remove them from the drug.
How do nitrosamine impurities form in the Maropitant drug?
Amines and nitrates are the root cause of forming nitrosamine impurities in Maropitant.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.