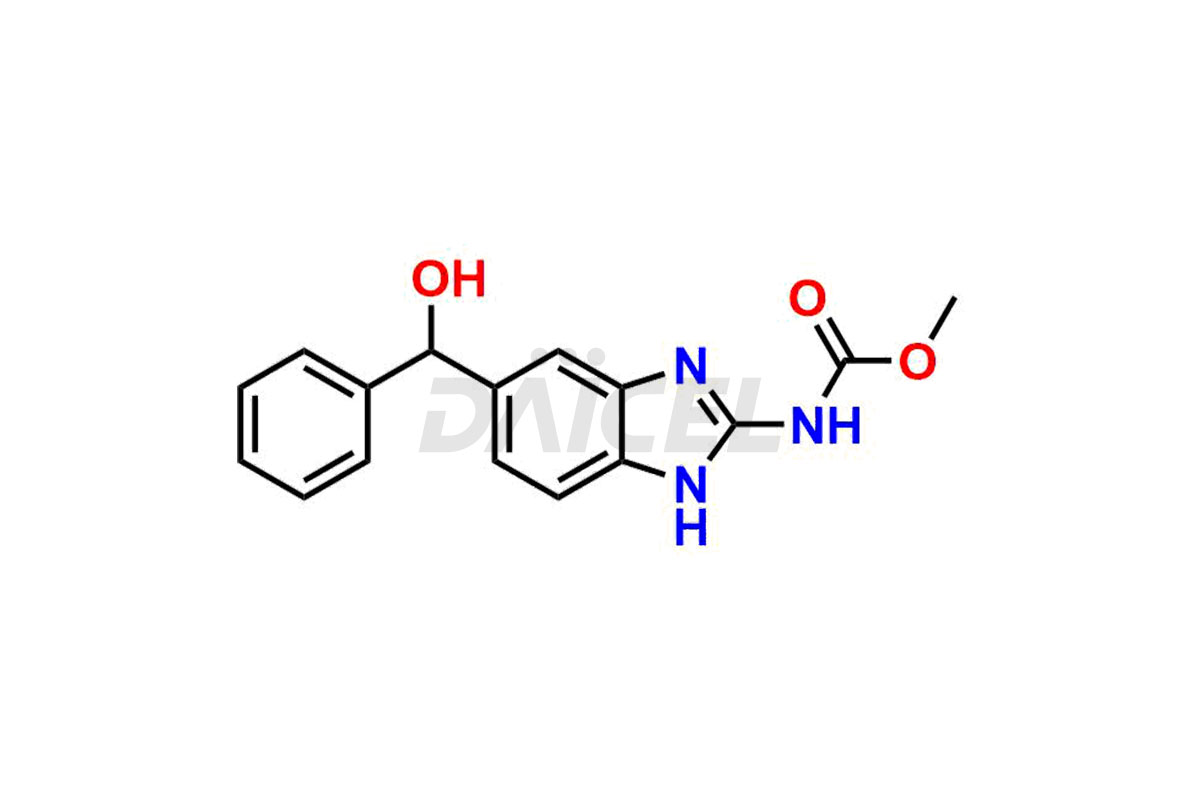
Mebendazole
General Information
Mebendazole Impurities and Mebendazole
Daicel Pharma offers the best-quality Mebendazole impurities, such as 5-Hydroxy Mebendazole Impurity. They are vital for evaluating Mebendazole quality, stability, and biological safety. Furthermore, Daicel Pharma specializes in the custom synthesis of Mebendazole impurities and ensures their worldwide delivery.
Mebendazole [CAS: 31431-39-7] is a benzimidazole derivative that treats gastrointestinal infections caused by parasites. These parasites include Enterobius vermicularis (pinworms), Ascaris lumbricoides (roundworms), Ancylostoma duodenale (hookworms), and Trichuris trichiura (whipworms). It is a broad-spectrum anthelminthic drug.
Mebendazole: Use and Commercial Availability
Mebendazole treats parasitic infections, including gastrointestinal diseases, in patients suffering from worm infestations. It also treats or controls nematode infections. Many manufacturers prepare Mebendazole under various brands. Mebendazole is available as an oral formulation under Vermox.
Mebendazole Structure and Mechanism of Action
The chemical name of Mebendazole is Methyl 5-benzoyl-2-benzimidazolecarbamate. The chemical formula for Mebendazole is C16H13N3O3, and its molecular weight is approximately 295.29 g/mol.
Mebendazole disrupts cellular tubulin formation in the helminth, resulting in degenerative changes in its intestine. Further, Mebendazole interrupts its glucose uptake and reproductive and digestive functions. It causes immobilization, stopping egg production and the helminth’s death.
Mebendazole Impurities and Synthesis
During Mebendazole synthesis1, impurities form that may affect the drug’s safety and efficacy. They form during the synthetic process, purification, or storage of Mebendazole. Therefore, Mebendazole impurities must be controlled and monitored throughout the drug’s development.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Mebendazole impurities, which includes 5-Hydroxy Mebendazole Impurity. We provide the CoA from a cGMP-compliant analytical facility. It gives complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We provide additional analytical data on request. Daicel Pharma can prepare any unidentified Mebendazole impurity or degradation product. In addition, Daicel Pharma offers highly purified, stable isotope-labeled standards of Mebendazole. We provide clients with complete characterization reports upon delivery.
References
- Van Gelder, Josephus L. H.; Raeymaekers, Alfons H. M.; Roevens, Leopold F. C., Benzimidazole carbamates, US3657267A, Jun 20, 1969, Janssen Pharmaceutica N.V. (https://www.lens.org/lens/search/patent/list?q=US3657267)
- Allan, R. J.; Goodman, H. T.; Watson, T. R., Two high-performance liquid chromatographic determinations for mebendazole and its metabolites in human plasma using a rapid Sep Pak C18 extraction, Journal of Chromatography, Biomedical Applications, Volume: 183,Issue: 3, Pages: 311-19, 1980 DOI: (10.1016/s0378-4347(00)81711-9)
Frequently Asked Questions
Which analytical methods identify and separate Mebendazole impurities?
The reverse-phase HPLC and UHPLC method helps to identify and separate Mebendazole impurities.
How do the Mebendazole impurities and degradation products form?
The raw materials, reagents, and catalysts that remain unreacted during synthesis and poor storage conditions may form Mebendazole impurities and degradation products.
Which analytical method can identify the degradation products of Mebendazole present in the drug?
HPLC method is the analytical method that can identify the degradation products of Mebendazole.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.