LOAD MORE
You're viewed 9 of 16 products
For evaluating the purity and safety of Melphalan, an active pharmaceutical ingredient, Daicel Pharma offers a customized synthesis of Melphalan impurity standards. These impurity standards include crucial compounds such as D-Isomer Melphalan, Dihydroxy Melphalan, Melphalan EP impurity-F, Melphalan impurity-8, Melphalan Methyl Ester, and more. Additionally, Daicel Pharma provides worldwide delivery options for Melphalan impurity standards.
Melphalan [CAS: 148-82-3], an alkylating agent, is derived from phenylalanine and nitrogen mustard. It exhibits antineoplastic activity and treats multiple myeloma and ovarian cancer through oral and parenteral administration.
The antineoplastic agent known as Melphalan functions as a bifunctional alkylating agent. It treats multiple myeloma, advanced ovarian adenocarcinoma, early and advanced breast cancer, childhood neuroblastoma, and polycythemia vera. It treats localized cases of malignant melanoma and soft-tissue sarcoma of the extremities. Melphalan is for regional arterial perfusion. The drug is available under the brand names Alkeran and Evomela.
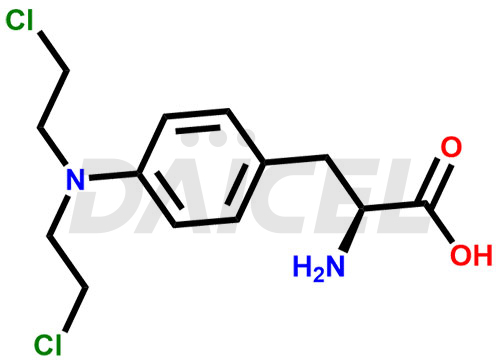
The chemical name of Melphalan is 4-[Bis(2-chloroethyl)amino]-L-phenylalanine. Its chemical formula is C13H18Cl2N2O2, and its molecular weight is approximately 305.20 g/mol.
Melphalan is an alkylating agent that binds at the N7 position of guanine.
Melphalan, a phenylalanine derivative of nitrogen mustard, may contain impurities that affect its purity and potency. Common Melphalan impurities include related substances, residual solvents, and degradation products. They can arise during the manufacturing or storage of the drug. Strict quality control measures help ensure that Melphalan meets regulatory standards for impurity levels. Monitoring and minimizing impurities in Melphalan is crucial to maintain its efficacy and safety.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for preparing Melphalan impurity standards, which include D-Isomer Melphalan, Dihydroxy Melphalan, Melphalan EP impurity-F, Melphalan impurity-8, Melphalan Methyl Ester, and more. Our Melphalan impurity standards have a detailed Certificate of Analysis (CoA) that provides a comprehensive characterization report. This report includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis1,2. Upon request, we give additional data like 13C-DEPT. Moreover, we can synthesize unknown Melphalan impurity standards and degradation products. Each delivery has a comprehensive characterization report.
Impurities in Melphalan are controlled through strict quality control measures during the drug manufacturing process. Good Manufacturing Practices (GMP) ensure they are minimized and meet regulatory standards. Additionally, stability studies assess impurity formation over the drug's shelf life.
Research on impurities in Melphalan is an ongoing process. Scientists and pharmaceutical companies continue to explore and develop improved analytical techniques, manufacturing processes, and impurity control strategies to enhance the quality and safety of Melphalan and other medications.
Water or Methanol is the solvent used when analyzing most impurities in Melphalan.
Melphalan impurities should be stored, at a controlled room temperature, usually between 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.