Menbutone
General Information
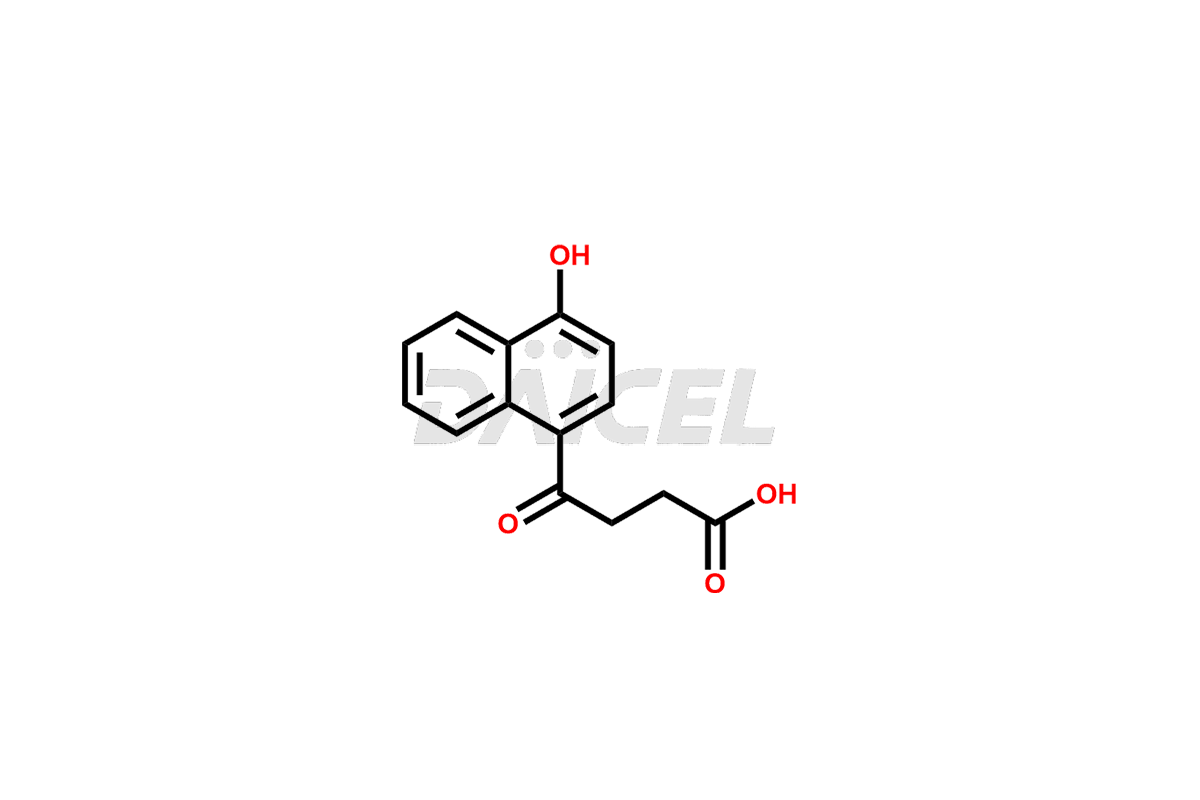
Menbutone Impurities and Menbutone
Daicel Pharma offers high-quality Menbutone impurities, such as 4-(4-hydroxynaphthalen-1-yl)-4-oxobutanoic acid and 4-(naphthalen-1-yloxy)-4-oxobutanoic acid. It is vital for evaluating Menbutone quality, stability, and biological safety. In addition, Daicel Pharma specializes in the custom synthesis of Menbutone impurities and ensures their worldwide delivery.
Menbutone [CAS: 3562-99-0] treats digestive disorders in animals. It is an oxybutyric acid derivative. Menbutone vitalizes hepato-digestive activity in sheep, goats, cattle, and pigs. A choleretic compound, Menbutone stimulates hepatic insufficiency in animals.
Menbutone: Use and Commercial Availability
Menbutone is a veterinary drug and a liver detoxifier. It acts on the transport and absorption of food in animals. It improves the gastroenteric movements and appetite of animals. In addition, it treats a wide range of digestive disorders in animals. Menbutone is administered parenterally (intravenous or intramuscular). In Europe, it is available under various trade names. Alvebuton and Genabil are some of the names of Menbutone.
Menbutone Structure and Mechanism of Action

The chemical name of Menbutone is 4-(4-Methoxynaphthalen-1-yl)-4-oxobutanoic acid. The chemical formula for Menbutone is C15H14O4 and its molecular weight is approximately 258.27 g/mol.
Menbutone increases the secretion of bile, pancreatic juice, and gastric acid to stimulate the digestive tract of animals.
Menbutone Impurities and Synthesis
While synthesizing Menbutone 1, impurities may form that affect the safety and efficacy of the drug. During the production and storage of Menbutone, impurities form. Thus, the control and monitoring of Menbutone impurities is essential in every stage of drug development.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Menbutone impurities, which includes 4-(4-hydroxynaphthalen-1-yl)-4-oxobutanoic acid and 4-(naphthalen-1-yloxy)-4-oxobutanoic acid. The issued CoA is from a cGMP-compliant analytical facility. It contains detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional data like 13C-DEPT and CHN on request. Daicel Pharma can make any unidentified Menbutone impurities or degradation products. In addition, Daicel Pharma offers highly purified isotope-labeled standards of Menbutone for bioanalytical research and BA/BE studies. We also provide a complete characterization report on delivery.
References
FAQ's
References
- Burtner, Robert R., Methods for producing alkoxynaphthoylalkanoic acids, US2623065A, Dec 23. 1952, G.D. Searle and Co.
- Lopez, Cristina; Diez, Raquel; Rodriguez, Jose M.; Sierra, Matilde; Garcia, Juan J.; Fernandez, Nelida; Diez, M. Jose; Sahagun, Ana M., Determination of Menbutone: Development and Validation of a Sensitive HPLC Assay according to the European Medicines Agency Guideline, Separations, Volume: 9, Issue: 4, Pages: 84, 2022 DOI: (10.3390/separations9040084)
Frequently Asked Questions
What is the temperature for storing Menbutone impurities?
The storage of Menbutone impurities is at 2-8°C.
How does chemical degradation of Menbutone impurities occur during drug development?
During Menbutone synthesis, various functional groups can undergo hydrolysis and oxidation to give Menbutone impurities. Further, purification steps and storage conditions form Menbutone impurities.
Which regulatory authorities are responsible for identifying Menbutone impurities?
The United States Food and Drug Administration (FDA), International Conference on Harmonization (ICH), and European Medicine Agency (EMA) are some of the regulatory authorities that identify and characterize Menbutone impurities.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.