Mephenesin
General Information
Mephenesin Impurities and Mephenesin
Daicel Pharma offers the best-quality Mephenesin impurities and labeled standards. They are vital for evaluating Mephenesin quality, stability, and biological safety. Furthermore, Daicel Pharma specializes in the custom synthesis of Mephenesin impurities and ensures their worldwide delivery.
Mephenesin [CAS: 59-47-2] is a glycerol ether compound. As a centrally-acting muscle relaxant, it reduces neuronal excitability and muscle spasticity. Mephenesin inhibits inward sodium and calcium currents in neurons.
Mephenesin: Use and Commercial Availability
Mephenesin is a muscle relaxant. It treats muscle spasticity in Parkinson’s disease. It also treats muscle spasms in other diseases, such as multiple sclerosis. It relieves painful muscle spasms. Many generic manufacturers prepare Mephenesin under various brands. Glotal, Myanesin, Myorelax, etc., are brands of Mephenesin. The route of administration differs for Mephenesin depending on the patient’s needs and health. It could be oral, subcutaneous, or intramuscular.
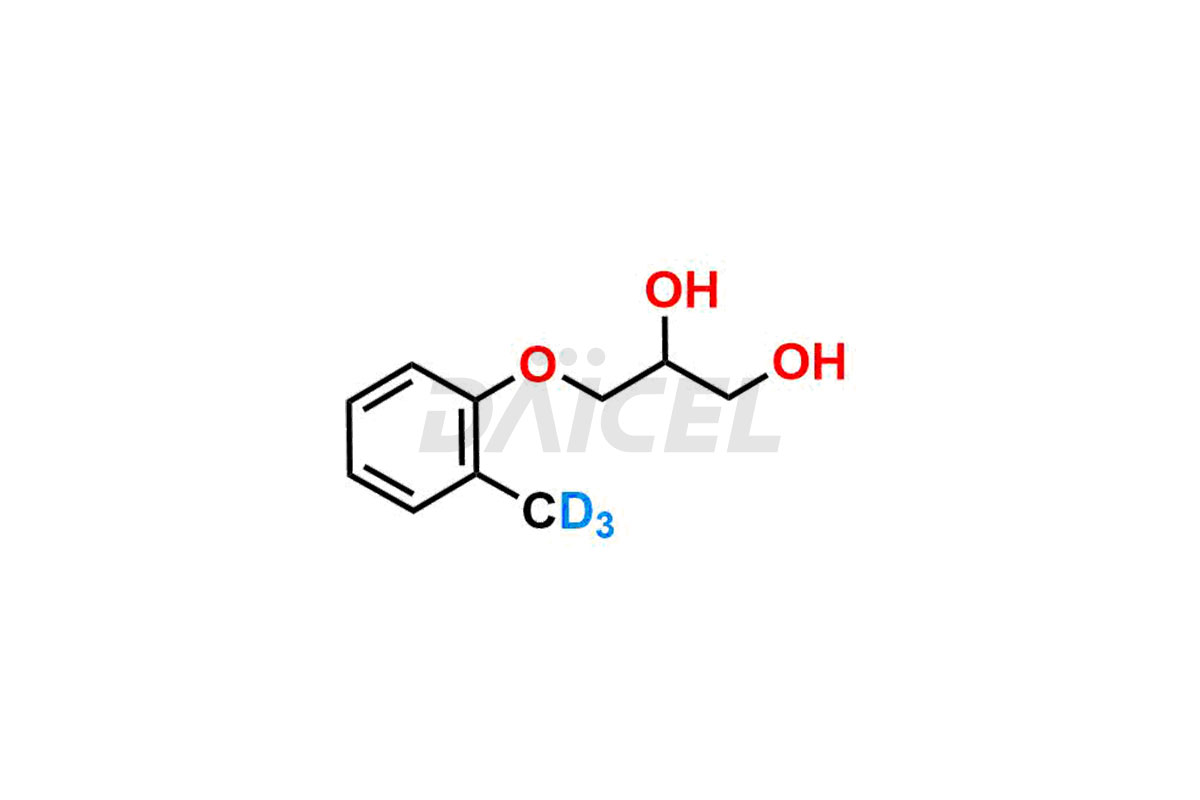
Mephenesin Structure and Mechanism of Action
The chemical name of Mephenesin is 3-(2-Methylphenoxy)-1,2-propanediol. The chemical formula for Mephenesin is C10H14O3, and its molecular weight is approximately 182.22 g/mol.
The precise mechanism of action of Mephenesin is unknown.
Mephenesin Impurities and Synthesis
During Mephenesin synthesis, impurities form that may affect the safety and efficacy of the drug. They form during the drug manufacturing, purification, or storage of Mephenesin. Therefore, Mephenesin impurities must be controlled and monitored throughout the drug’s development.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Mephenesin impurities and labeled standards. We provide the CoA from a cGMP-compliant analytical facility. It gives complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPCL1,2. We provide additional analytical data on request. Daicel Pharma can prepare any unidentified Mephenesin impurity or degradation product. In addition, Daicel Pharma offers a highly purified, stable deuterium-labeled standard, Mephenesin Labelled Standard. The clients of Daicel Pharma can expect a complete characterization report on delivery.
References
- Guinebault, P.; Colafranceschi, C.; Bianchetti, G., Determination of mephenesin in plasma by high-performance liquid chromatography with fluorimetric detection, Journal of Chromatography, Volume: 507, Pages: 221-5, 1990 DOI: (10.1016/s0021-9673(01)84197-2)
- Dias, L.; Mallya, R.; Patani, K., Development and validation of new RP-HPLC method for estimation of Mephenesin and Ibuprofen, International Journal of Pharmaceutical Sciences and Research, Volume: 7, Issue: 12, Pages: 4971-4977, 2016 DOI: (13040/ijpsr.0975-8232.7(12).4971-77)
Frequently Asked Questions
Which analytical method identifies and separates Mephenesin impurities?
HPLC is the analytical method that helps to identify and separate Mephenesin impurities.
How do the Mephenesin impurities and degradation products form?
The raw materials, reagents, and catalysts that remain unreacted during synthesis and poor storage conditions may form Mephenesin impurities and degradation products.
Why is it necessary to eliminate Mephenesin impurities and degradation products from the drug?
Mephenesin impurities and degradation products can affect the drug quality, safety, and efficacy; hence, their removal is essential.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.