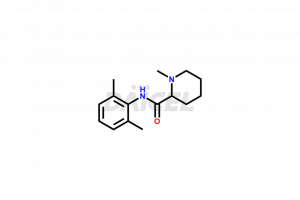
Mepivacaine
General Information
Mepivacaine Impurities and Mepivacaine
Daicel Pharma offers excellent-quality Mepivacaine impurities, such as Mepivacaine EP Impurity-D. They are vital for evaluating Mepivacaine quality, stability, and biological safety. In addition, Daicel Pharma specializes in the custom synthesis of Mepivacaine impurities and ensures worldwide delivery.
Mepivacaine [CAS: 96-88-8] is a carboxamide derivative and a local anesthetic. It is similar in properties to lidocaine, but its effect lasts for a longer duration. Mepivacaine inhibits neuronal sodium channels and is a nerve blocker when administering anesthesia.
Mepivacaine: Use and Commercial Availability
Mepivacaine is available under Carbocaine as an injectable formulation. Many brands market Mepivacaine. It is a regional or local anesthetic. It is used in dental procedures in patients by nerve block or infiltration. Further, Mepivacaine can produce analgesia in patients. The dosage of Mepivacaine varies depending on the method, duration, and patient’s physical condition.
Mepivacaine Structure and Mechanism of Action
The chemical name of Mepivacaine is N-(2,6-Dimethylphenyl)-1-methyl-2-piperidinecarboxamide. The chemical formula for Mepivacaine is C15H22N2O, and its molecular weight is approximately 246.35 g/mol.
Mepivacaine stops the formation and conduction of nerve impulses. It raises the electric excitation threshold in the nerve and decreases nerve impulse propagation.
Mepivacaine Impurities and Synthesis
During Mepivacaine synthesis1, impurities form that may affect drug safety and efficacy. They form during drug manufacture, purification, or storage. The impurities must be controlled and monitored throughout the drug development process.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Mepivacaine impurities, which includes Mepivacaine EP Impurity-D. We provide the CoA from a cGMP-compliant analytical facility. It gives complete characterization data2 such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We provide additional analytical data on request. Daicel Pharma can prepare any unidentified Mepivacaine impurity or degradation product. In addition, Daicel Pharma offers highly purified, stable isotope-labeled standards of Mepivacaine. The clients of Daicel Pharma can expect a complete characterization report on delivery.
References
- N-alkyl piperidine monocarboxylic acid amides and n-alkyl pyrrolidine -a-monocarboxylic acid amides, GB770129A, Mar 3, 1957, Aktiebolaget Bofors (https://www.lens.org/lens/search/patent/list?q=GB770129A)
- Pratt, Edward L.; Warrington, Horace P.; Grego, J., Gas-chromatographic determination of mepivacaine in blood with a note on other local anesthetics, Anesthesiology, Volume: 28, Issue: 2, Pages: 432-7,1967 DOI: (10.1097/00000542-196703000-00028)
Frequently Asked Questions
What is the analytical method that identifies and analyzes Mepivacaine impurities?
RP-HPLC helps identify and analyze Mepivacaine impurities.
How do Mepivacaine degradation products form?
Mepivacaine degradation products form due to improper storage conditions and exposure to light, moisture, temperature, etc.
Why should Mepivacaine impurities be removed from the drug?
If Mepivacaine impurities are present in the drug, they can affect its safety, quality, and efficacy. Hence, their removal is essential.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.