Methimazole
General Information
Methimazole Impurities and Methimazole
Daicel Pharma offers superior-quality Methimazole impurities and Labeled Standards. These impurities are essential for evaluating Methimazole quality, stability, and biological safety. In addition, Daicel Pharma specializes in the custom synthesis of Methimazole impurities and ensures their worldwide delivery.
Methimazole [CAS: 60-56-0] is a medicine to treat hyperthyroidism, specifically in cases where surgical or radioactive iodine therapy is unsuitable. It is an active metabolite of Carbimazole. It decreases the production of thyroid hormones thyroxine (T4) and triiodothyronine (T3). They regulate body growth, development, and metabolism.
Methimazole: Use and Commercial Availability
Methimazole treats hyperthyroidism, wherein the body produces excessive thyroid hormones. It also treats Graves’ disease, an autoimmune disease that affects the thyroid gland. Methimazole treats patients with thyroid storm or thyrotoxicosis. In addition, it treats patients with toxic nodular goiters unsuitable for surgery. It is available as an oral formulation under Tapazole.
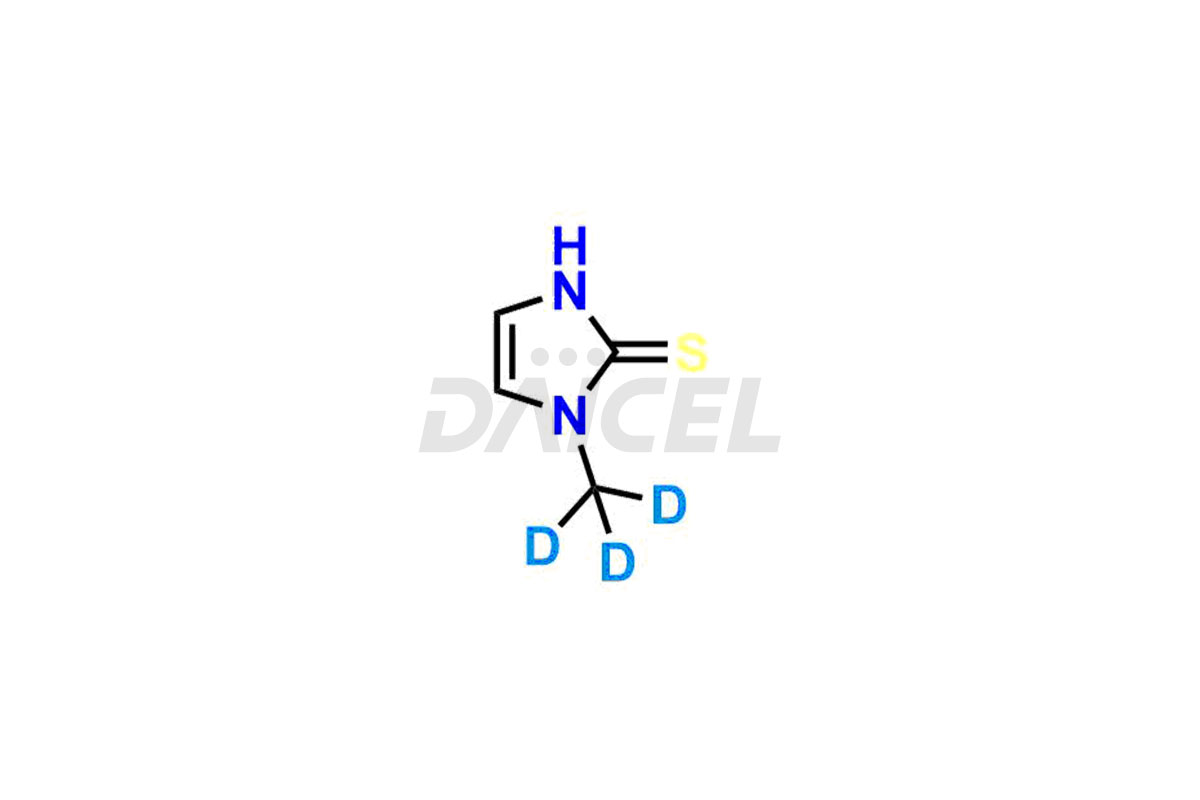
Methimazole Structure and Mechanism of Action
The chemical name of Methimazole is 3-methyl-1H-imidazole-2-thione . The chemical formula for Methimazole is C4H6N2S, and its molecular weight is approximately 114.17 g/mol.
Methimazole prevents thyroid generation in the thyroid gland. It disturbs the enzymatic process intervened by thyroid peroxidase, which causes iodination of tyrosine residues in thyroglobulin. It inhibits the synthesis of T4 and T3 thyroid hormones.
Methimazole Impurities and Synthesis
While synthesizing Methimazole1, impurities may form that will affect drug safety and efficacy. They form during the synthetic process, storage, or purification of Methimazole. Manufacturers can control and monitor Methimazole impurities to improve the drug’s safety, efficacy, and storage.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Methimazole impurities and Labeled Standards. The CoA given to clients is from a cGMP-compliant analytical facility, which has the complete characterization data1 such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional spectral data on request. Daicel Pharma can prepare any unidentified Methimazole impurity or degradation product. In addition, Daicel Pharma offers a highly purified, stable deuterium-labeled standard, the Methimazole Labelled Standard. Daicel Pharma provides a complete characterization report accompanying the delivery.
References
- Stenlake, John B.; Williams, William David; Skellern, G. G., Development and comparison of thin-layer chromatographic and gas-liquid chromatographic methods for measurement of methimazole in rat urine, Journal of Chromatography, Volume: 53, Issue: 2, Pages: 285-91, 1970 DOI: (1016/s0021-9673(01)98469-9)
- Skellern, G. G.; Knight, B. I.; Stenlake, J. B., Improved method for the determination of methimazole in plasma by high-performance liquid chromatography, Journal of Chromatography, Volume: 124, Issue: 2, Pages: 405-10, 1976 DOI: (10.1016/s0021-9673(00)89761-7)
Frequently Asked Questions
Which conditions cause the maximum degradation of Methimazole?
Oxidative hydrolytic conditions are responsible for the maximum degradation of Methimazole.
Which analytical techniques can identify the degradation products of Methimazole?
The ultraperformance liquid chromatography and Quadrupole time-of-flight tandem mass spectrometry (UPLC-Q-TOF MS/MS) techniques can identify the degradation products of Methimazole.
Why is it necessary to remove nitrosamine Methimazole impurities from the drug product?
Nitrosamine Methimazole impurities can be harmful to human health and may be carcinogenic. Hence, their removal from the drug is essential.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.