Midodrine
General Information
Midodrine Impurities and Midodrine
Daicel Pharma offers superior-quality Midodrine impurities and labeled standards. These impurities are essential for evaluating Midodrine quality, stability, and biological safety. In addition, Daicel Pharma specializes in the custom synthesis of Midodrine impurities and ensures their worldwide delivery.
Midodrine [CAS: 42794-76-3] is an ethanolamine derivative that treats hypotension. The US FDA has declared it as an orphan drug for managing orthostatic hypotension. As a sympathomimetic drug, it stimulates adrenergic receptors and tightens blood vessels, increasing blood pressure.
Midodrine: Use and Commercial Availability
Midodrine treats low blood pressure conditions and orthostatic hypotension in patients. In addition, it acts as a vasoconstrictor agent. Its active metabolite, Desglymidodrine, constricts peripheral arterial and venous vessels. Further, it does not cross the blood-brain barrier, thus avoiding some central nervous system (CNS) side effects. It is available as an oral formulation under Proamantine.
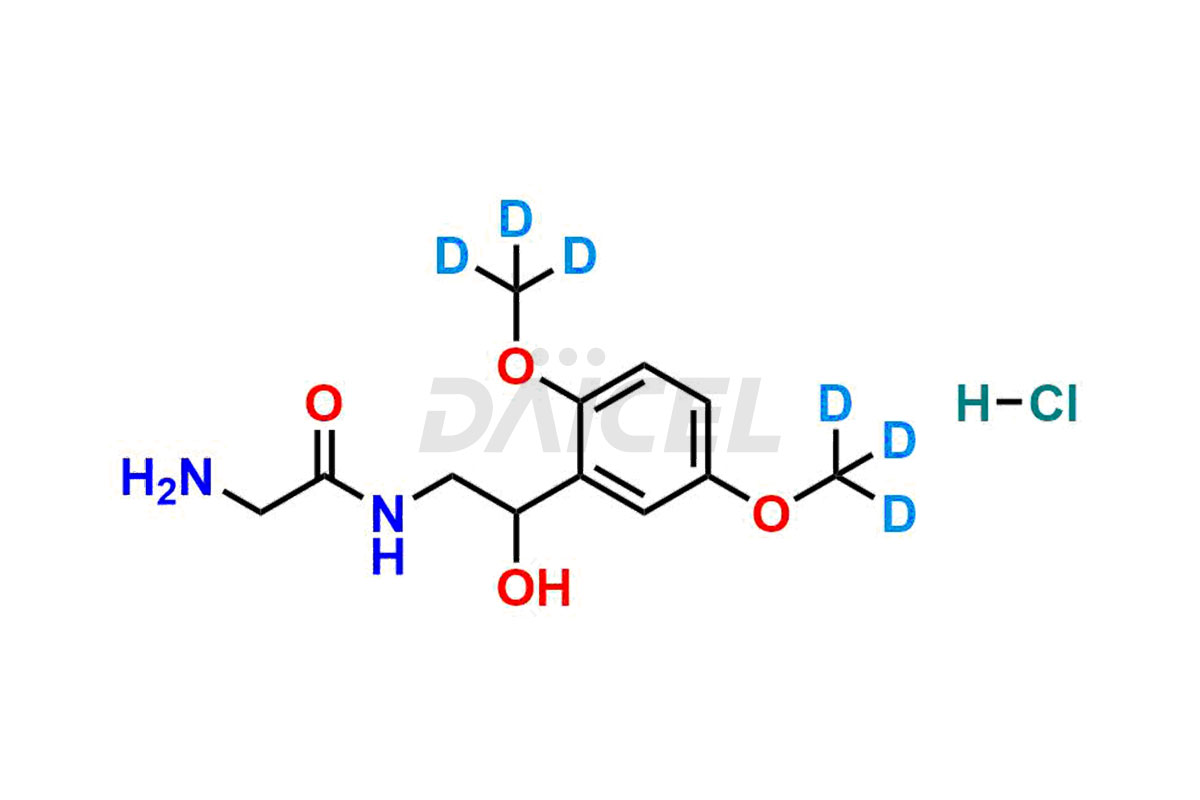
Midodrine Structure and Mechanism of Action
The chemical name of Midodrine is 2-Amino-N-[2-(2,5-dimethoxyphenyl)-2-hydroxyethyl]acetamide. The chemical formula for Midodrine is C12H18N2O4, and its molecular weight is approximately 254.28 g/mol.
Midodrine metabolizes to its active metabolite, desglymidodrine. It activates the alpha-adrenergic receptors of the arteriolar and venous vasculature. Midodrine increases vascular tone and elevation of blood pressure.
Midodrine Impurities and Synthesis
While synthesizing Midodrine1, impurities may form that will affect drug safety and efficacy. They form during the synthetic process, storage, or purification of Midodrine. Manufacturers can control and monitor Midodrine impurities to improve the drug’s safety, efficacy, and storage.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Midodrine impurities and labeled standards. The CoA given to clients is from a cGMP-compliant analytical facility. It has the complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional spectral data on request. Daicel Pharma can prepare any unidentified Midodrine impurity or degradation product. In addition, Daicel Pharma offers a highly purified, stable deuterium-labeled standard, Midodrine Labelled Standard. Daicel Pharma provides a complete characterization report accompanying the delivery.
References
FAQ's
References
- Zoelss, Gerhard, 2-amino-n-method for producing (beta-hydroxy-phenaethyl)-acetamide-derivatives and of their salts, DE2506110C2, Feb 13, 1975, Lentia G.m.b.H. Chem. und Pharm. Erzeugnisse-Industriebedarf, Federal Republic of Germany (https://www.lens.org/lens/search/patent/list?q=DE2506110)
- Posch, Werner; Lindner, Wolfgang, Quantification of midodrine and its active metabolite in plasma using a high-performance liquid chromatography column switching technique, Biomedical Chromatography, Volume: 3, Issue: 4, Pages: 153-6, 1989 DOI: (10.1002/bmc.1130030403)
Frequently Asked Questions
What causes the formation of nitrosamine Midodrine impurities?
Contamination by raw materials, reagents, recovered solvents, catalysts, and the quenching process, is responsible for the formation of the nitrosamine Midodrine impurities.
Which analytical technique identifies Midodrine degradation products?
A chiral RP-HPLC method helps identify Midodrine degradation products.
Why is it necessary to remove nitrosamine Midodrine impurities?
The nitrosamine Midodrine impurities can be carcinogenic and affect human health. Hence, it is essential to remove them from the drug.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.