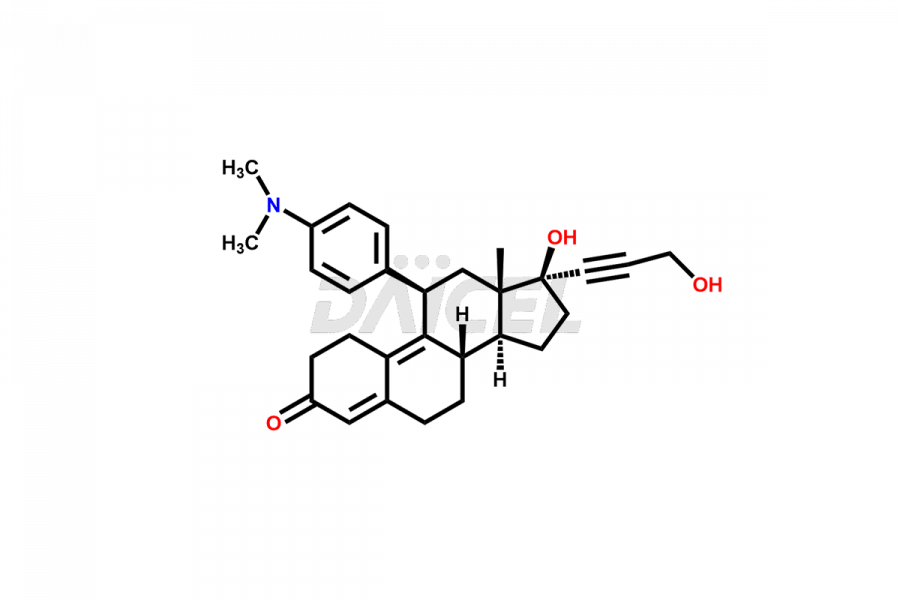
Mifepristone
General Information
Mifepristone Impurities and Mifepristone
Daicel Pharma offers high-quality Mifepristone impurities, such as Hydroxy Mifepristone and N-Desmethyl Mifepristone. They are vital for evaluating Mifepristone quality, stability, and biological safety. Furthermore, Daicel Pharma specializes in the custom synthesis of Mifepristone impurities and ensures their worldwide delivery.
Mifepristone [CAS: 84371-65-3], a synthetic steroid, is a progesterone antagonist. RU486, or Mifepristone, is prescribed for medical termination of intrauterine pregnancy. It is a cortisol receptor blocker that controls hyperglycemia and hypercortisolism in Cushing’s syndrome patients. In addition, these patients have type 2 diabetes or cannot tolerate glucose.
Mifepristone: Use and Commercial Availability
Mifepristone has two FDA-approved uses. With Misoprostol, Mifepristone aids in pregnancy termination. It manages and treats hyperglycemia in patients with Cushings syndrome symptoms. It also can be used as a contraceptive and in treating endometriosis and breast and ovarian cancer. Further, it appears to have the potential to treat neuropsychiatric disorders. In the US, Korlym and Mifeprex are Mifepristone trade names. In Europe, Mifegyne is a brand among other different brands.
Mifepristone Structure and Mechanism of Action


The chemical name of Mifepristone is 17β-Hydroxy-11β-[4-(dimethylamino)-phenyl]-17α-(prop-1-ynyl)-estra-4,9-dien-3-one. The chemical formula for Mifepristone is C29H35NO2 and its molecular weight is approximately 429.59 g/mol.
Mifepristone blocks cortisol at the glucocorticoid receptor (GR-II). It affects the hypothalamic-pituitary-adrenal axis, thus controlling hyperglycemia in patients. In addition, it interrupts the progesterone hormone. It increases prostaglandins, leading to menstrual bleeding, endometrial disruption, and termination of pregnancy.
Mifepristone Impurities and Synthesis
During the synthesis of Mifepristone 1, impurities may form that may affect the safety and efficacy of the drug. These impurities form during Mifepristone synthesis, storage, or degradation. As a result, impurities must be controlled and monitored throughout the drug’s lifecycle.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Mifepristone impurities, which includes Hydroxy Mifepristone and N-Desmethyl Mifepristone. The issued CoA is from a cGMP-compliant analytical facility. It contains detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional data like 13C-DEPT and CHN on request. Daicel Pharma can prepare any unidentified Mifepristone impurity or degradation product. In addition, Daicel Pharma offers highly purified deuterium-labeled standards of Hydroxy Mifepristone D6 for bioanalytical research and BA/BE studies. We also provide a complete characterization report on delivery.
References
FAQ's
References
- Teutsch, Jean Georges; Costerousse, Germain; Philibert, Daniel; Deraedt, Roger, Novel steroids, US4386085A, Jul 16, 1982, Roussel-UCLAF, France
- Guo, Zhiyong; Chu, Chengding; Yin, Gengxin; He, Mingli; Fu, Keqin; Wu, Jianli, An HPLC method for the determination of ng mifepristone in human plasma, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 832, Issue: 2, Pages: 181-184, 2006 DOI: (10.1016/j.jchromb.2005.12.019)
Frequently Asked Questions
How do you separate Mifepristone Impurities from the drug substance?
TLC and HPLC are the analytical methods to separate Mifepristone Impurities from the drug substance.
Why is it vital to control Mifepristone Impurities?
Controlling Mifepristone Impurities is essential for the drug to be safe, effective, and stable.
How can the formation of Mifepristone Impurities be minimized during synthesis?
The formation of Mifepristone Impurities can be minimized by optimizing the synthetic process, using high-quality raw materials, and maintaining strict quality control measures.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.