Milnacipran
General Information
Milnacipran Impurities and Milnacipran
Daicel Pharma offers the best-quality Milnacipran impurities and labeled standards. They are vital for evaluating Milnacipran quality, stability, and biological safety. Furthermore, Daicel Pharma specializes in the custom synthesis of Milnacipran impurities and ensures their worldwide delivery.
Milnacipran [CAS: 92623-85-3] belongs to a class of drugs, like serotonin and norepinephrine reuptake inhibitors (SNRIs). Levomilnacipran is the enantiomer of Milnacipran. It has fewer side effects compared to other tricyclic anti-depressants. It does not show neurological changes in the alpha1-adrenergic pathway.
Milnacipran: Use and Commercial Availability
Milnacipran is a drug that treats patients with major depressive disorder (MDD) and fibromyalgia. It also treats pain disorders, including neuropathies. It improves fibromyalgia symptoms in patients. It is available as an oral formulation under Savella in the U.S. It is approved by the US FDA for the treatment of fibromyalgia. Milnacipran is sold globally under different brand names. In Japan, it is available as Toledomin and in Mexico, as Dalcipran.
Milnacipran Structure and Mechanism of Action
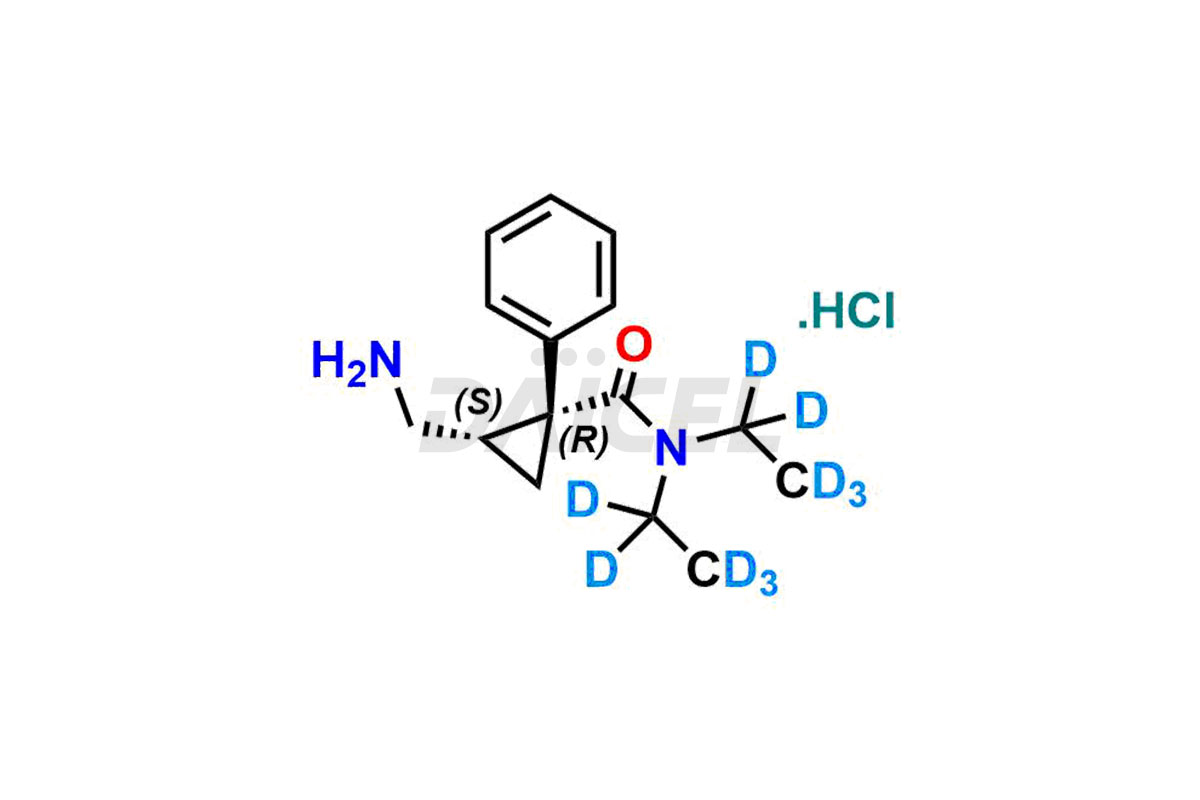
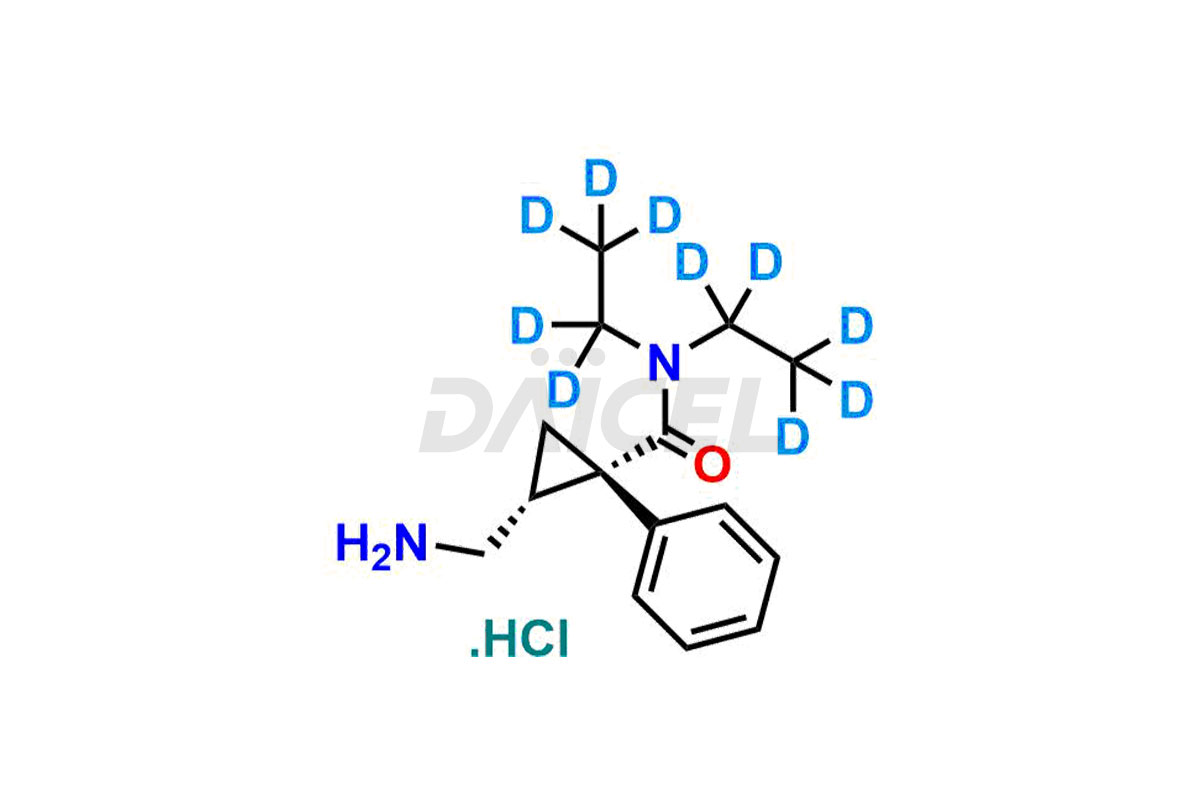
The chemical name of Milnacipran is (1R,2S)-rel-2-(Aminomethyl)-N, N-diethyl-1-phenylcyclopropanecarboxamide. The chemical formula for Milnacipran is C15H22N2O, and its molecular weight is approximately 246.35 g/mol.
The precise mechanism of action of Milnacipran is unclear.
Milnacipran Impurities and Synthesis
Impurity formation during the synthesis of Milnacipran1 may affect the drug’s safety and efficacy. These impurities form during the manufacturing process, purification, or storage of Milnacipran. Therefore, Milnacipran impurities must be controlled and monitored throughout the drug’s development.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Milnacipran impurities and labeled standards. We provide the CoA from a cGMP-compliant analytical facility. It gives complete characterization data2 such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We provide additional analytical data on request. Daicel Pharma can prepare any unidentified Milnacipran impurity or degradation product. In addition, Daicel Pharma offers highly purified, stable isotope-labeled standards, such as Dextro-milnacipran.HCl-D10, and Levo-milnacipran.HCl–D10. We provide a complete characterization report to clients on delivery.
References
FAQ's
References
- Hascoet, Pierre; Cousse, Henri, Industrial process for producing Midalcipran, FR2581060B1, Apr 25, 1985, P. F. Medicament, France (https://www.lens.org/lens/search/patent/list?q=FR2581060A1)
- Puozzo, Christian; Filaquier, Christian; Zorza, Gregoire, Determination of milnacipran, a serotonin and noradrenaline reuptake inhibitor, in human plasma using liquid chromatography with spectrofluorimetric detection, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 806, Issue: 2, Pages: 221-228, 2004 DOI: (10.1016/j.jchromb.2004.03.063)
Frequently Asked Questions
What are the analytical methods to characterize Milnacipran impurities?
IR, LC-MS, 1H NMR, and 13C NMR are the analytical methods that characterize Milnacipran impurities.
How do process-related Milnacipran impurities form in the drug?
Contaminated raw materials, reagents, and unreacted catalysts during synthesis form process-related Milnacipran impurities.
Why is it necessary to remove Milnacipran impurities from the drug?
Milnacipran impurities can affect drug safety and quality and pose health risks, so it is necessary to remove them from the drug.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.