LOAD MORE
You're viewed 9 of 13 products
Daicel Pharma synthesizes high-quality Minocycline impurities like 4-EpiMinocycline, 7-Monomethyl Minocycline, Minocycline Dehydro analogue, 12-Imino-delta-Minocycline, and more, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Minocycline. Moreover, Daicel Pharma offers custom synthesis of Minocycline impurities and delivers them globally.
Minocycline [CAS: 10118-90-8] is a second-generation tetracycline antibiotic that is semi-synthetic. It has many applications in treating various infectious and non-infectious diseases. The drug has comparable anti-infectious properties to other tetracyclines.
Minocycline is available in various forms and treats numerous medical conditions. Oral and topical forms manage inflammatory acne lesions. The drug is also available in oral and intravenous formulations to treat infections caused by susceptible microorganisms. Some microorganisms treated by Minocycline include Mycoplasma pneumoniae, Chlamydia trachomatis, Yersinia pestis, and Escherichia coli. Minocycline is available under several brand names, including Amzeeq, Arestin, Dynacin, Minocin, Minocycline Hydrochloride, Minolira, Solodyn, Ximino, and Zilxi.
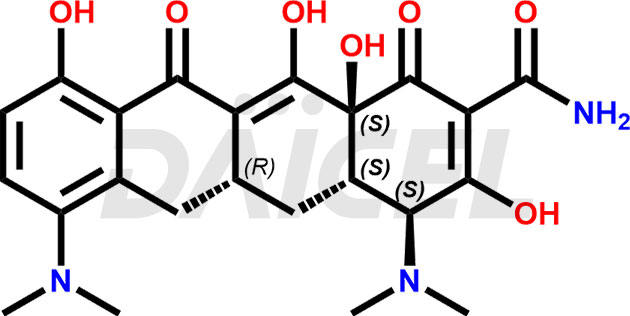
The chemical name of Minocycline is (4S,4aS,5aR,12aS)-4,7-Bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide. Its chemical formula is C23H27N3O7, and its molecular weight is approximately 457.5 g/mol.
Minocycline exerts its antimicrobial effects by inhibiting bacterial protein synthesis.
Minocycline can undergo degradation, resulting in the formation of impurities. The impurities that form during the degradation of Minocycline include 4-epiMinocycline and other related compounds. The impurities are due to pH, temperature, and storage conditions. Controlling these factors during the manufacturing1, storage, and use of Minocycline can help minimize impurity formation and maintain product quality.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Minocycline impurity standards, 4-EpiMinocycline, 7-Monomethyl Minocycline, Minocycline Dehydro analogue, 12-Imino-delta-Minocycline, and more. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We also provide 13C-DEPT and CHN if requested. We give a complete characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Minocycline impurity or degradation product.
High-performance liquid chromatography (HPLC), LC-MS, and UP-LC are commonly used analytical techniques for detecting and quantifying impurities in Minocycline.
The Impurities in Minocycline can affect the pharmacokinetics through absorption, distribution, metabolism, and elimination.
Methanol is a solvent that helps in analyzing most of the Minocycline impurities.
Minocycline impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.