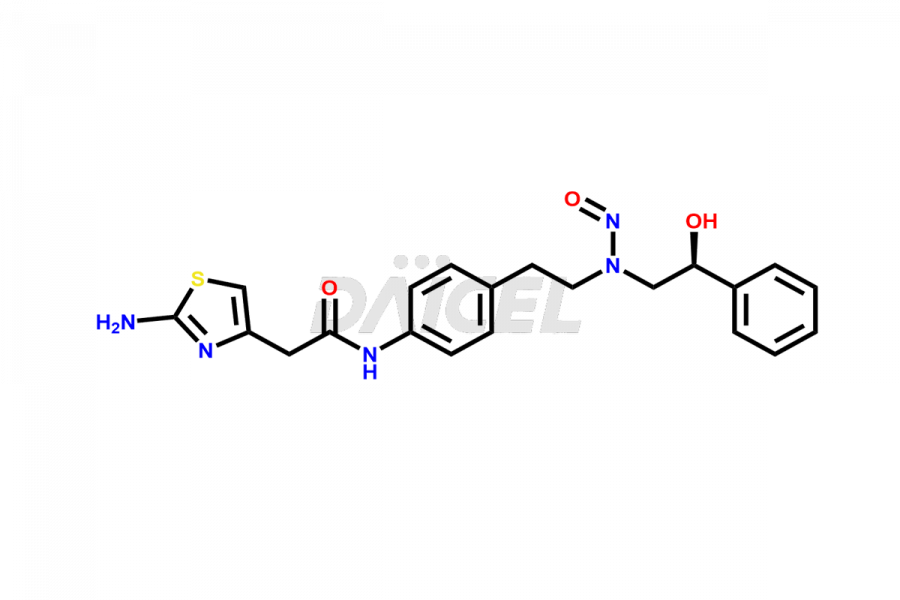
Mirabegron
References
- Maruyama, Tatsuya; Suzuki, Takayuki; Onda, Kenichi; Hayakawa, Masahiko; Moritomo, Hiroyuki; Kimizuka, Tetsuya; Matsui, Tetsuo, Amide Derivatives Or Salts Thereof, Yamanouchi Pharmaceutical Co., Ltd., Japan, EP1028111B1, May 12, 2004
- van Teijlingen, Raymond; Meijer, John; Takusagawa, Shin; van Gelderen, Marcel; van den Beld, Cas; Usui, Takashi, Development and validation of LC-MS/MS methods for the determination of mirabegron and its metabolites in human plasma and their application to a clinical pharmacokinetic study, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 887-888, Pages: 102-111,2012
Frequently Asked Questions
Can Mirabegron impurities be removed from the drug product?
Mirabegron impurities removal from the drug product involves various manufacturing processes such as recrystallization, filtration, or chromatography. However, the impurity removal process needs to be validated and effective without affecting the safety and efficacy of the drug product.
Are there any safety concerns related to Mirabegron impurities?
High levels of Mirabegron impurities can harm patients. Therefore, it is necessary to control the impurity levels to ensure drug safety.
Which solvent helps in the analysis of Mirabegron impurities?
Methanol is a solvent used for analyzing many impurities in Mirabegron.
What are the temperature conditions required to store Mirabegron impurities?
Mirabegron impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.