Monomethyl fumarate
General Information
Monomethyl fumarate Impurities and Monomethyl fumarate
Daicel Pharma offers the best-quality Monomethyl fumarate impurities and labeled standards. They are vital for evaluating Monomethyl fumarate quality, stability, and biological safety. Furthermore, Daicel Pharma specializes in the custom synthesis of Monomethyl fumarate impurities and ensures their worldwide delivery.
Monomethyl fumarate [CAS: 2756-87-8] is a dicarboxylic acid monoester compound. It neutralizes reactive oxygen species and reduces inflammation associated with multiple sclerosis. It has antioxidant and immunomodulatory effects. Further, it promotes the repair and regeneration of myelin, the protective sheath around nerve fibers affected in multiple sclerosis.
Monomethyl fumarate: Use and Commercial Availability
Monomethyl fumarate is available as an oral formulation under Bafiertam. It treats multiple sclerosis, which affects the central nervous system. It helps relieve symptoms of inflammation, demyelination, and axonal degeneration. It treats autoimmune diseases, such as psoriasis, Parkinson’s disease, amyotrophic lateral sclerosis, and Huntington’s disease.
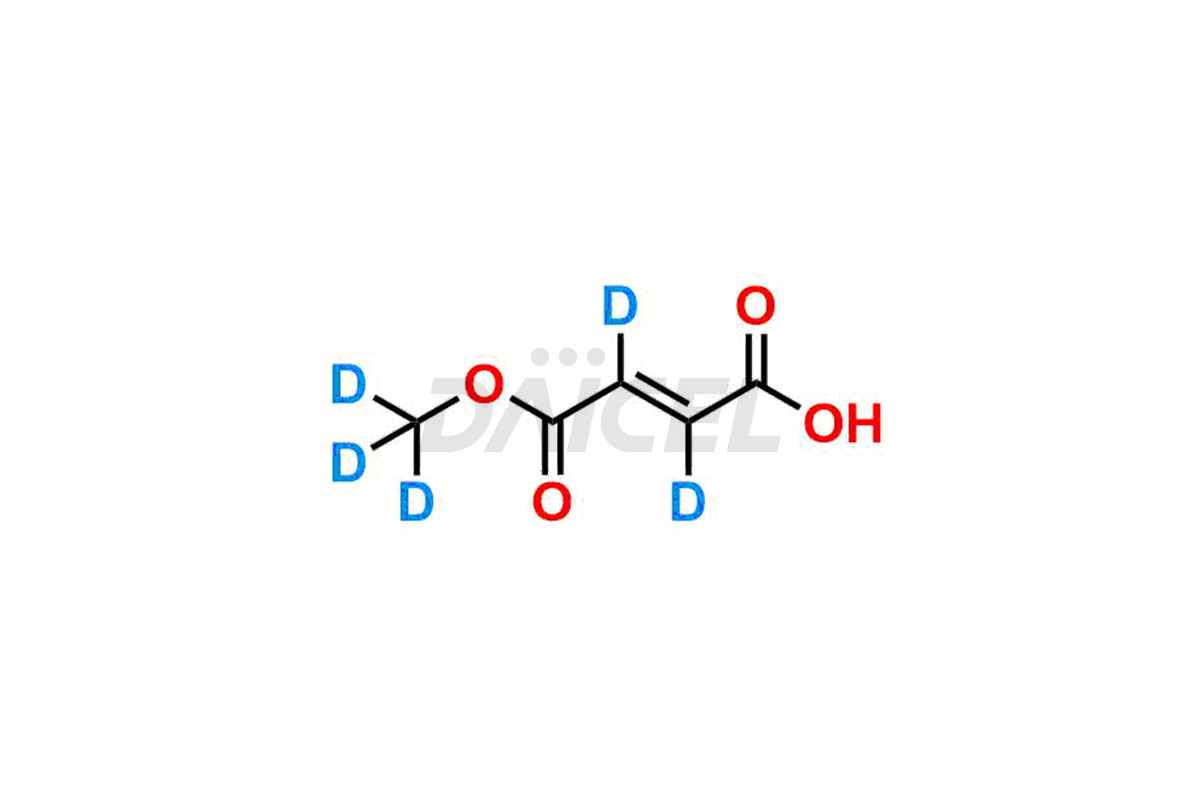
Monomethyl fumarate Structure and Mechanism of Action
The chemical name of Monomethyl fumarate is (E)-4-Methoxy-4-oxo-but-2-enoic acid. The chemical formula for Monomethyl fumarate is C5H6O4, and its molecular weight is approximately 130.10 g/mol.
Monomethyl fumarate activates the Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathway for the cellular response to oxidative stress. Nf2 in the cell nucleus initiates antioxidant and cytoprotective gene transcription.
Monomethyl fumarate Impurities and Synthesis
Impurities form during the manufacture of monomethyl fumarate that may affect the drug’s safety and efficacy. These impurities form during the manufacturing process, purification, or storage of Monomethyl fumarate. Therefore, Monomethyl fumarate impurities must be controlled and monitored throughout the drug’s development.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Monomethyl fumarate impurities and labeled standards. We provide the CoA from a cGMP-compliant analytical facility. It gives complete characterization data1 such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We provide additional analytical data on request. Daicel Pharma can prepare any unidentified Monomethyl fumarate impurity or degradation product. In addition, Daicel Pharma offers a highly purified, stable deuterium-labeled standard, Monomethyl fumarate Labelled Standard. We provide a complete characterization report to clients on delivery.
References
- Imam Pasha, S.; Varanasi, Murali Balaram; Mohammed, Ibrahim, Bioanalysis of monomethyl fumarate in human plasma by a sensitive and rapid LC-MS/MS method and its pharmacokinetic application LC-MS/MS determination of monomethyl fumarate in human plasma, Journal of Pharmaceutical and Biomedical Analysis, Volume: 146, Pages: 109-116, 2017 DOI: (1016/j.jpba.2017.08.015)
- Jirovsky, David; Wiegrebe, Wolfgang, HPLC-Analysis of Fumarates in Biological Matrices, Monatshefte fuer Chemie, Volume: 135, Issue: 12, Pages: 1563-1568, 2004 DOI: (10.1007/s00706-004-0230-6)
Frequently Asked Questions
What are the possible reasons for the formation of Monomethyl fumarate impurities?
Starting materials, reagents, unreacted catalysts during synthesis, and poor storage conditions may form Monomethyl fumarate impurities.
Why is it necessary to remove Monomethyl fumarate impurities from the drug?
Monomethyl fumarate impurities can affect drug safety and quality and pose health risks, so it is necessary to remove them from the drug.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.