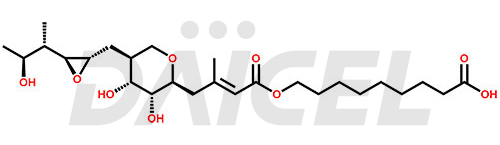
Mupirocin
General Information
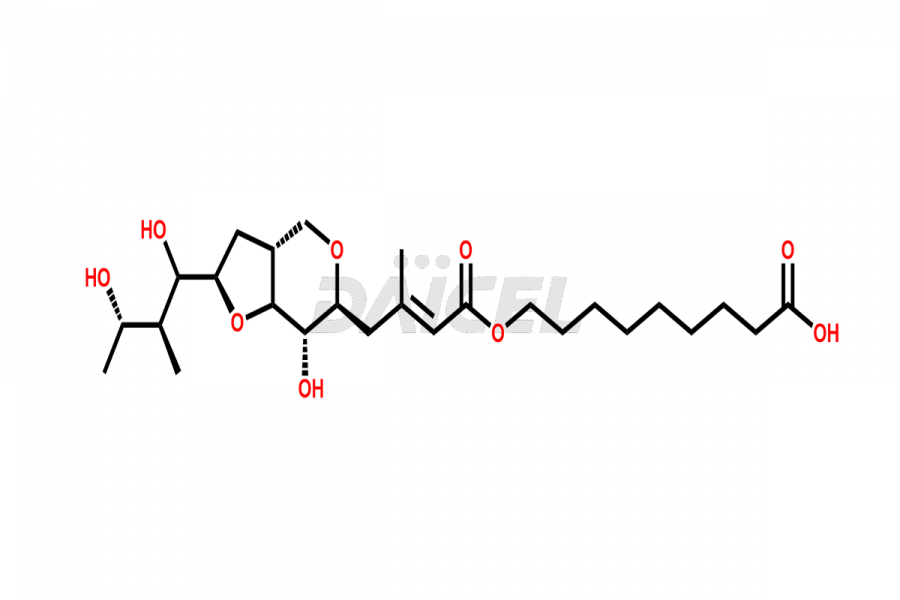
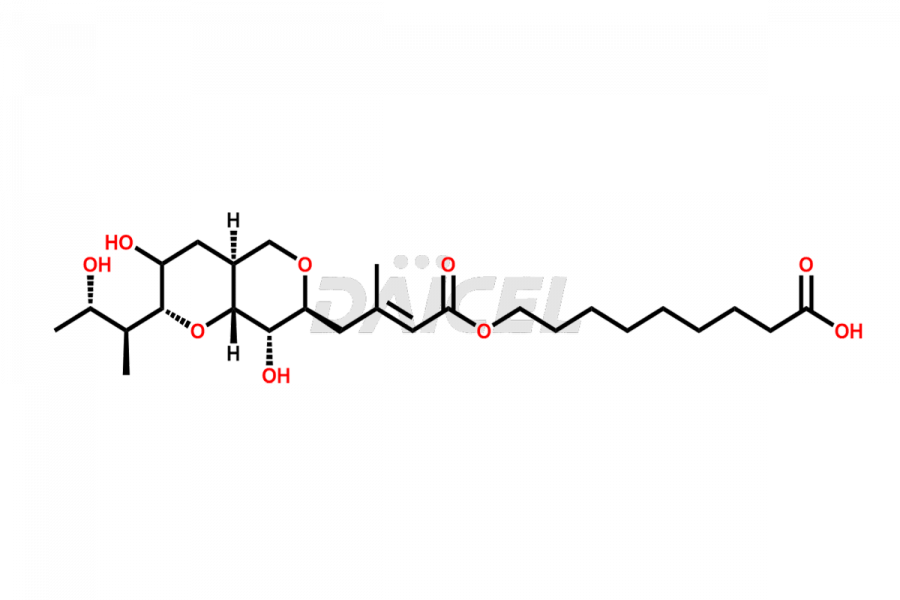
Mupirocin Impurities and Mupirocin
Daicel Pharma is a trusted provider of quality Mupirocin impurity standards, including Mupirocin EP impurity D, Mupirocin EP impurity E, and Mupirocin EP impurity F. They are crucial in evaluating the quality, stability, and safety of the active pharmaceutical ingredient, Mupirocin. Furthermore, Daicel Pharma customizes Mupirocin impurities to meet client specifications. With global shipping capabilities, these impurities can be conveniently delivered to customers worldwide, providing unparalleled convenience.
Mupirocin [CAS: 12650-69-0] is a topical antibiotic commonly used to treat impetigo and bacterial skin infections.
Mupirocin: Use and Commercial Availability
Mupirocin is a topical antibacterial that treats skin infections caused by bacteria. It is effective against impetigo, folliculitis, and minor skin infections and can help eliminate methicillin-resistant Staphylococcus aureus (MRSA) bacteria. It treats human skin infections caused by S. aureus, Neisseria spp., and Haemophilus influenza.
Mupirocin is available under the brand name Bactroban and Centany, which contains the active ingredient Mupirocin.
Mupirocin Structure and Mechanism of Action
The chemical name of Mupirocin is 9-(((E)-4-((2S,3R,4R,5S)-3,4-dihydroxy-5-(((2S,3S)-3-((2S,3S)-3-hydroxybutan-2-yl)oxiran-2-yl)methyl)tetrahydro-2H-pyran-2-yl)-3-methylbut-2-enoyl)oxy)nonanoic acid. Its chemical formula is C26H44O9, and its molecular weight is approximately 500.6 g/mol.
Mupirocin specifically binds to bacterial isoleucyl transfer-RNA synthetase and inhibits bacterial protein synthesis.
Mupirocin Impurities and Synthesis
Mupirocin impurities can arise during synthesis due to the storage or use of specific raw materials and intermediates in manufacturing. These impurities encompass related compounds, degradation products, and process impurities. Stringent quality control measures and analytical methods are crucial to ensure the purity and safety of Mupirocin for patient use.
Daicel Pharma provides a comprehensive Certificate of Analysis (CoA) for Mupirocin impurity standards, such as Mupirocin EP impurity D, Mupirocin EP impurity E, and Mupirocin EP impurity F. The CoA includes detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. Additionally, upon delivery, a complete 13C-DEPT is also provided. Daicel Pharma possesses the technology and expertise to synthesize any unknown Mupirocin impurity or degradation product.
References
FAQ's
References
- Luk, Kong; Clayton, John Peter; Rogers, Norman Harold, Antibacterial compounds, Beecham Group Ltd., United Kingdom, US4102901A, July 25, 1978
- Porter, Regina S.; Chen, Ted K., High-performance liquid chromatographic analysis of mupirocin in polyethylene glycols 400 and 3350 using dual ultraviolet and evaporative light scattering detection, Journal of Chromatography A, Volume: 732, Issue: 2, Pages: 399-402, 1996
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.