Nadolol
General Information
Nadolol Impurities and Nadolol
Daicel Pharma offers superior-quality Nadolol impurities, such as N-Nitroso-Nadolol. These impurities are essential for evaluating Nadolol quality, stability, and biological safety. In addition, Daicel Pharma specializes in the custom synthesis of Nadolol impurities and ensures their worldwide delivery.
Nadolol [CAS: 42200-33-9] is a β- adrenergic receptor blocker that treats high blood pressure in patients. It has long-term use in managing angina pectoris. It blocks the effect of catecholamine on β-1 receptors. Its beta-blocking activity can block isoproterenol-induced tachycardia.
Nadolol: Use and Commercial Availability
Nadolol manages hypertension, angina, and various cardiovascular diseases. Cardiovascular diseases include stroke, heart failure, and coronary heart disease. In addition, it helps in lowering free thyroid hormone levels in the bloodstream. It improves thyrotoxicosis symptoms in patients. It also helps in preventing migraine headaches. It also aids patients with liver cirrhosis. Nadolol is available as an oral formation under Corgard.
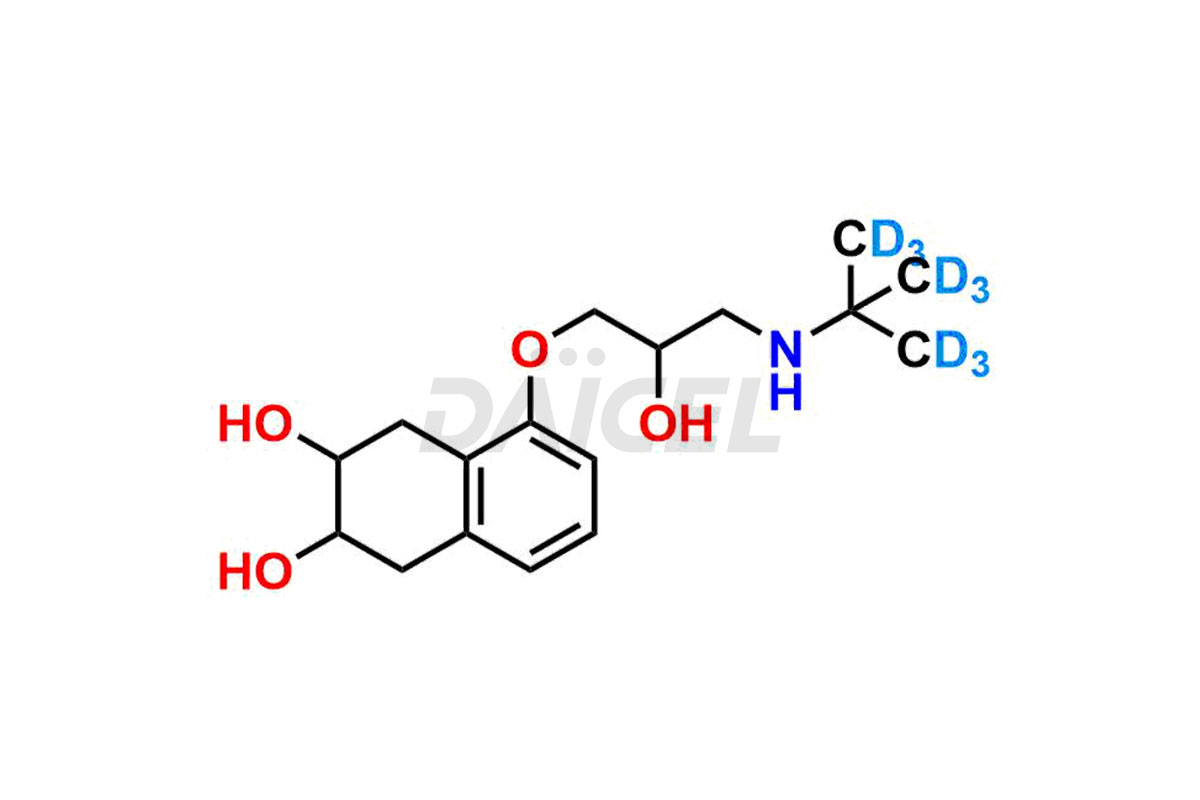
Nadolol Structure and Mechanism of Action
The chemical name of Nadolol is 5-[3-[(1,1-Dimethylethyl)amino]-2-hydroxypropoxy]-1,2,3,4-tetrahydro-2,3-naphthalenediol. The chemical formula for Nadolol is C17H21NO4, and its molecular weight is approximately 309.40 g/mol.
Nadolol stops the β-1 receptors in the vascular smooth muscles and heart. It decreases peripheral vascular resistance and lowers systolic and diastolic blood pressure.
Nadolol Impurities and Synthesis
While synthesizing Nadolol 1, impurities may form that will affect drug safety and efficacy. They form during the synthetic process, storage, or purification of Nadolol. Manufacturers can control and monitor Nadolol impurities to improve the drug’s safety, efficacy, and storage.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Nadolol impurities, which includes D N-Nitroso-Nadolol. The CoA given to clients is from a cGMP-compliant analytical facility, which has the complete characterization data2 such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We give additional spectral data on request. Daicel Pharma can prepare any unidentified Nadolol impurity or degradation product. In addition, Daicel Pharma offers a highly purified, stable deuterium-labeled standard, Nadolol Labelled Standard. Daicel Pharma provides a complete characterization report accompanying the delivery.
References
FAQ's
References
- Hauck, Frederic P.; Cimarusti, Christopher M.; Narayanan, Venkatachala L., Tetrahydronaphthyloxy-aminopropanols and salts thereof, US3935267A, Dec 1, 1971, R. Squibb and Sons, Inc. (https://www.lens.org/lens/search/patent/list?q=US3935267)
- Funke, Phillip T.; Malley, Mary F.; Ivashkiv, Eugene; Cohen, Allen I., Determination of serum nadolol levels by GLC-selected ion monitoring mass spectrometry: comparison with a spectrofluorometric method, Journal of Pharmaceutical Sciences, Volume: 67, Issue: 5, Pages: 653-7, 1978 DOI: (10.1002/jps.2600670521)
Frequently Asked Questions
What causes the formation of Nadolol impurities?
Side reactions and by-products formed during the synthetic process are the source of Nadolol impurities.
Which analytical method helps in identifying Nadolol impurities in the drug?
Ultra-high performance liquid chromatography coupled with mass spectrometry (UHPLC–MS) is the analytical method can identify Nadolol impurities in the drug.
Why is it essential to remove nitrosamine Nadolol impurities from the drug?
The nitrosamine Nadolol impurities can be carcinogenic and affect human health. Hence, it is essential to remove them from the drug.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.