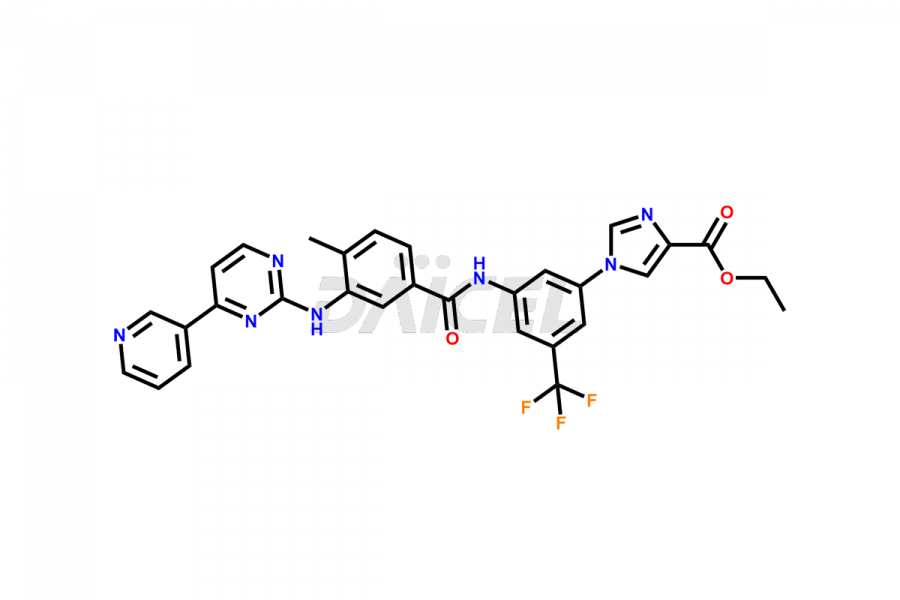
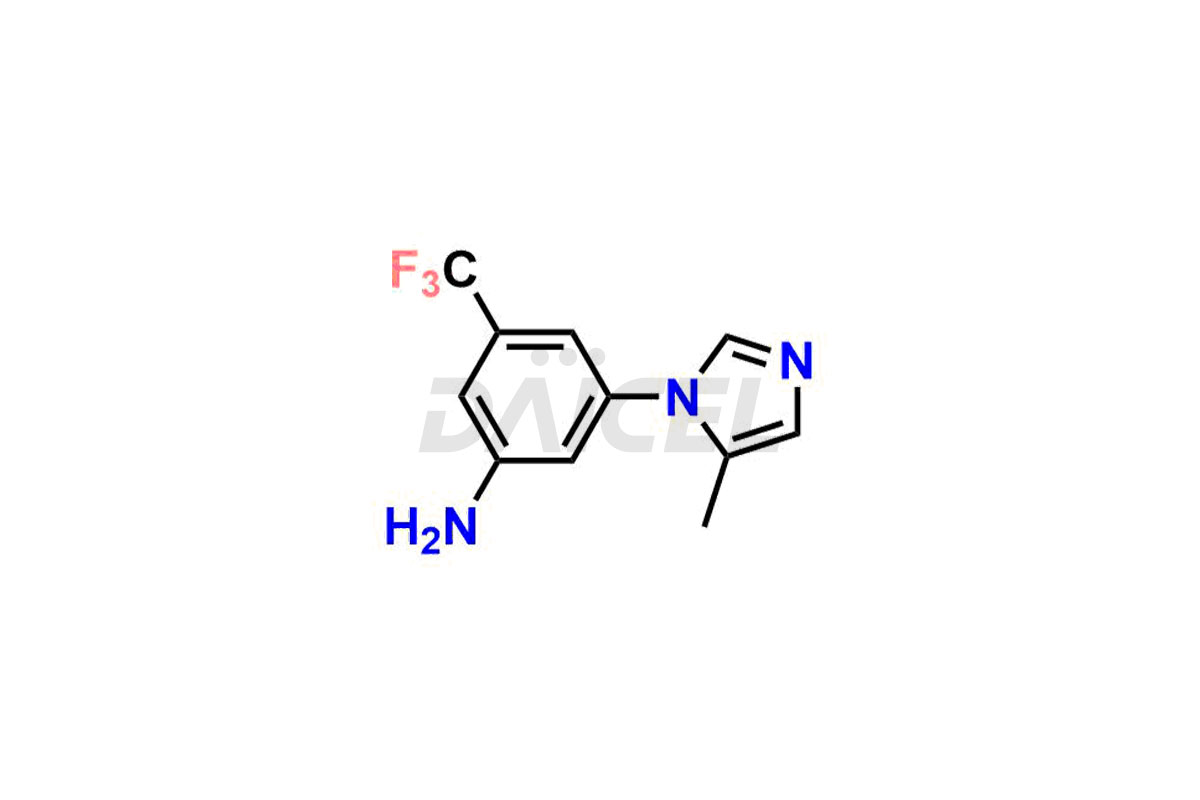
Nilotinib
References
- Breitenstein, Werner; Furet, Pascal; Jacob, Sandra; Manley, Paul William, Inhibitors Of Tyrosine Kinases, Novartis A.-G., Switzerland, EP1532138B1, November 19, 2008
- Pursche, S.; Ottmann, O. G.; Ehninger, G.; Schleyer, E., High-performance liquid chromatography method with ultraviolet detection for the quantification of the BCR-ABL inhibitor nilotinib (AMN107) in plasma, urine, culture medium and cell preparations, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 852, Issue: 1-2, Pages: 208-216, 2007
Frequently Asked Questions
How are Nilotinib Impurities controlled?
Nilotinib impurities' control is through strict quality control measures during manufacturing. Regulatory authorities set specific limits for impurity levels that must be adhered to.
Can Nilotinib Impurities affect its effectiveness?
Nilotinib impurities can affect its effectiveness by altering the drug's pharmacokinetics, pharmacodynamics, or stability. It is crucial to control and monitor impurities so that the drug maintains its therapeutic potency and delivers the desired therapeutic outcomes.
Are generic versions of Nilotinib tested for Impurities?
Nilotinib generics are thoroughly checked for impurities to assure their quality, safety, and efficacy. Comparative studies and thorough quality control help guarantee that generic versions are equal regarding purity and drug efficacy.
What are the temperature conditions required to store Nilotinib Impurities?
Nilotinib impurities should be stored at a controlled room temperature between 2-8°C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.