LOAD MORE
You're viewed 9 of 18 products
Daicel Pharma offers high-graded Nintedanib impurities such as (Z)-3-(1-hydroxypropylidene)-2-oxo-1-Propionlindoline-6-carboxylate, Acetyl impurity of Nintedanib CRS, Methyl Impurity of Nintedanib CRS, N-Acetyl Nintedanib Impurity, Nintedanib acid impurity, and more. These impurities are essential for evaluating Nintedanib quality, stability, and safety. Furthermore, Daicel Pharma provides a custom synthesis of Nintedanib impurities and distributes them internationally to fulfill the clientele’s needs.
Nintedanib [CAS: 656247-17-5] is a tyrosine kinase inhibitor treating Idiopathic Pulmonary Fibrosis (IPF) and chronic fibrosing interstitial lung diseases.
Nintedanib treats idiopathic pulmonary fibrosis (IPF) and delays the decline of pulmonary function in individuals with systemic sclerosis-related interstitial lung disease. It treats progressive chronic fibrosing interstitial lung disorders. Nintedanib is available under the brand name Ofev.
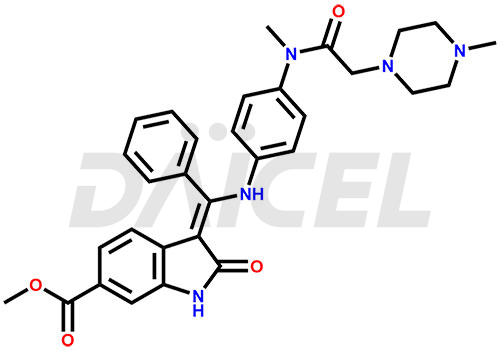
The chemical name of Nintedanib is Methyl (Z)-3-[[[4-(N-methyl-2-(4-methylpiperazin-1-yl)acetamido)phenyl)amino)(phenyl)methylene)-2-oxoindoline-6-carboxylate. Its chemical formula is C31H33N5O4, and its molecular weight is approximately 539.6 g/mol.
Nintedanib inhibits multiple receptor tyrosine kinases (RTKs) such as platelet-derived growth factor receptor (PDGFR) α and β, vascular endothelial growth factor receptor (VEGFR) 1-3, fibroblast growth factor receptor (FGFR) 1-3, etc. It binds with the adenosine triphosphate (ATP) binding pocket of receptors and prevents the intracellular signaling for transformation, migrations, and proliferation of fibroblasts vital for IPF pathology.
Nintedanib impurities refer to unwanted substances or by-products that may be present in the drug, Nintedanib. They can arise during the drug’s synthetic process1, storage, or degradation. Controlling and monitoring impurities helps ensure Nintedanib quality, safety, and efficacy.
Daicel provides a Certificate of Analysis (CoA) of Nintedanib impurity standards like (Z)-3-(1-hydroxypropylidene)-2-oxo-1-Propionlindoline-6-carboxylate, Acetyl impurity of Nintedanib CRS, Methyl Impurity of Nintedanib CRS, N-Acetyl Nintedanib Impurity, Nintedanib acid impurity, and more. The CoA is generated from a cGMP-compliant analytical facility and includes comprehensive characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. If requested, we give additional characterization data, such as 13C-DEPT. Daicel Pharma can synthesize unknown Nintedanib impurities or degradation products and supply labeled compounds to evaluate the effectiveness of generic Nilotinib. Each delivery comes with a complete characterization report. Daicel Pharma offers Nintedanib-D3, deuterium-labeled Nintedanib standards for bioanalytical research and BA/BE tests.
The impurities do not necessarily imply harm, but their levels and nature need careful regulation. Health authorities set specific limits for impurity levels based on safety and efficacy considerations.
Impurities in Nintedanib can affect its effectiveness by altering the drug's pharmacokinetics, pharmacodynamics, or stability. Controlling them ensures the drug maintains its therapeutic potency and delivers the desired outcome.
Analytical techniques such as High-Performance Liquid Chromatography (HPLC), Mass Spectrometry (MS) help detect and quantify Nintedanib impurities. These methods enable accurate identification and measurement of impurity levels.
Nintedanib impurities should be stored at a controlled room temperature between 2-8°C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.