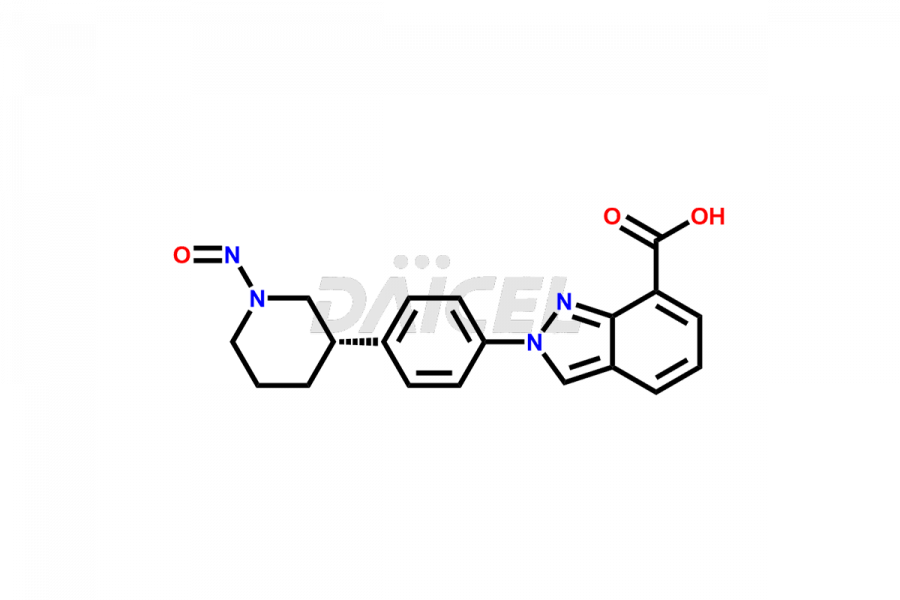
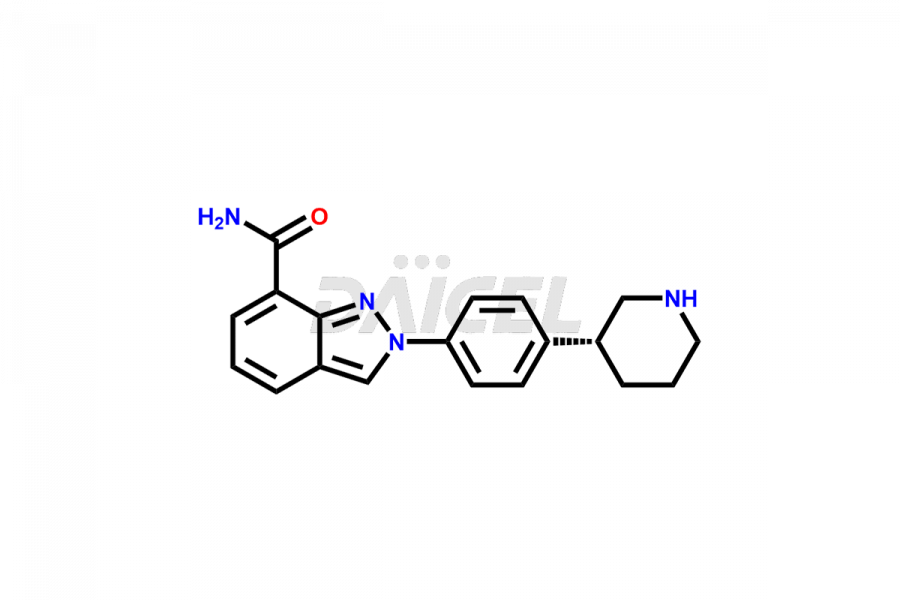
Niraparib
General Information
Niraparib Impurities and Niraparib
Daicel Pharma offers high-quality Niraparib impurities, such as (S)-tert-Butyl 3-(4-aminophenyl)Piperidine-1-Carboxylate, methyl 3-[(E)-(2-(dimethylamino)ethenyl]-2-nitrobenzoate, Niraparib Enantiomer, and tert-butyl (R)-3-(4-aminophenyl)Piperidine-1-Carboxylate. They are vital for evaluating Niraparib quality, stability, and biological safety. Furthermore, Daicel Pharma specializes in the custom synthesis of Niraparib impurities and ensures their worldwide delivery.
Niraparib [CAS: 1038915-60-4] is an aromatic, fused azabicyclic compound that treats ovarian or fallopian tube cancer. It is a part of the maintenance therapy in patients suffering from primary peritoneal, recurrent fallopian tube, or ovarian cancer. It is a PARP inhibitor with the potential to treat breast cancer.
Niraparib: Use and Commercial Availability
As a poly (ADP ribose) polymerase inhibitor, Niraparib treats women with recurrent ovarian cancer. In addition, Niraparib is effective against breast cancer as it decreases tumor growth in human cells with mutated BRCA1/2. It is administered orally to adult patients. Zejula is the brand name of Niraparib.
Niraparib Structure and Mechanism of Action


The chemical name of Niraparib is 2-[4-(3S)-3-Piperidinylphenyl]-2H-indazole-7-carboxamide. The chemical formula for Niraparib is C19H20N4O, and its molecular weight is approximately 320.39 g/mol.
Niraparib blocks PARP or poly (ADP ribose) polymerase enzymes that help in DNA repair.
Niraparib Impurities and Synthesis
During the synthesis of Niraparib1, impurities may form that may affect the safety and efficacy of the drug. These impurities form during the synthesis, storage, or degradation of Niraparib. As a result, Niraparib impurities must be controlled and monitored throughout the drug’s lifecycle.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Niraparib impurities, which includes (S)-tert-Butyl 3-(4-aminophenyl)Piperidine-1-Carboxylate, methyl 3-[(E)-(2-(dimethylamino)ethenyl]-2-nitrobenzoate, Niraparib Enantiomer, and tert-butyl (R)-3-(4-aminophenyl)Piperidine-1-Carboxylate. The issued CoA is from a cGMP-compliant analytical facility. It contains detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional data like 13C-DEPT and CHN on request. Daicel Pharma can prepare any unidentified Niraparib impurity or degradation product. In addition, Daicel Pharma offers highly purified isotope-labeled standards of Niraparib for bioanalytical research and BA/BE studies. We also provide a complete characterization report on delivery.
References
FAQ's
References
- Jones, Philip; Ontoria Ontoria, Jesus Maria; Scarpelli, Rita; Schultz-Fademrecht, Carsten, Amide substituted indazoles as poly(ADP-ribose)polymerase (PARP) inhibitors, WO2008084261A1, July 17, 2008, Istituto Di Ricerche Di Biologia Molecolare P. Angeletti SpA, Italy
- van Andel, L.; Zhang, Z.; Lu, S.; Kansra, V.; Agarwal, S.; Hughes, L.; Tibben, M. M.; Gebretensae, A.; Rosing, H.; Schellens, J. H. M.; et al, Liquid chromatography-tandem mass spectrometry assay for the quantification of niraparib and its metabolite M1 in human plasma and urine, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 1040, Pages: 14-21, 2017 DOI: (10.1016/j.jchromb.2016.11.020)
Frequently Asked Questions
What are the conditions under which Niraparib impurities form in the drug product?
High temperature, humidity, and improper storage conditions cause the formation of Niraparib impurities in the drug product.
What happens if Niraparib impurities remain in the drug?
If Niraparib impurities remain in the drug, it will cause harmful effects to patients and may even lead to death.
Why is it essential to have an impurity profile for Niraparib impurities?
It is essential to have an impurity profile to detect any changes in the drug substance or modifications in raw materials that will impact the drug’s safety and efficacy.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.