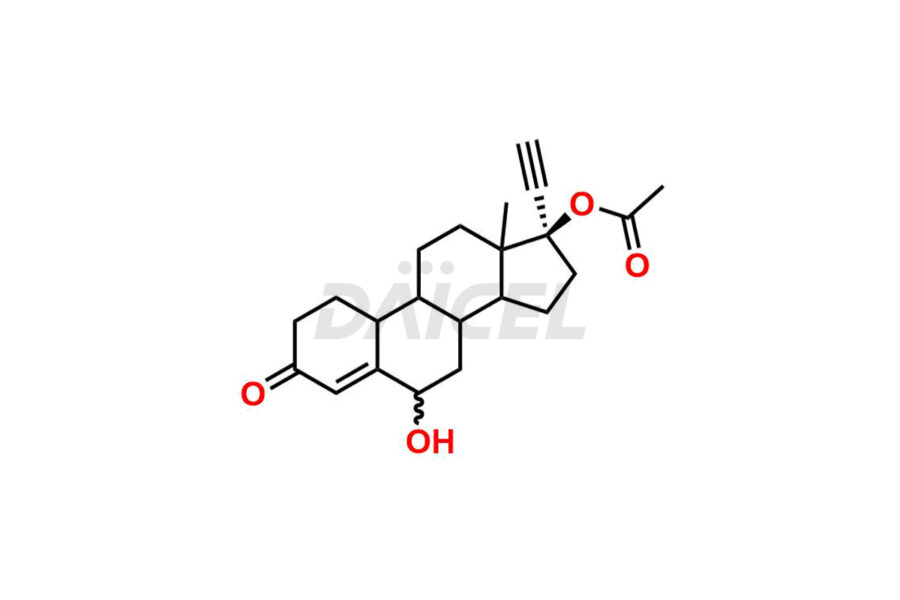
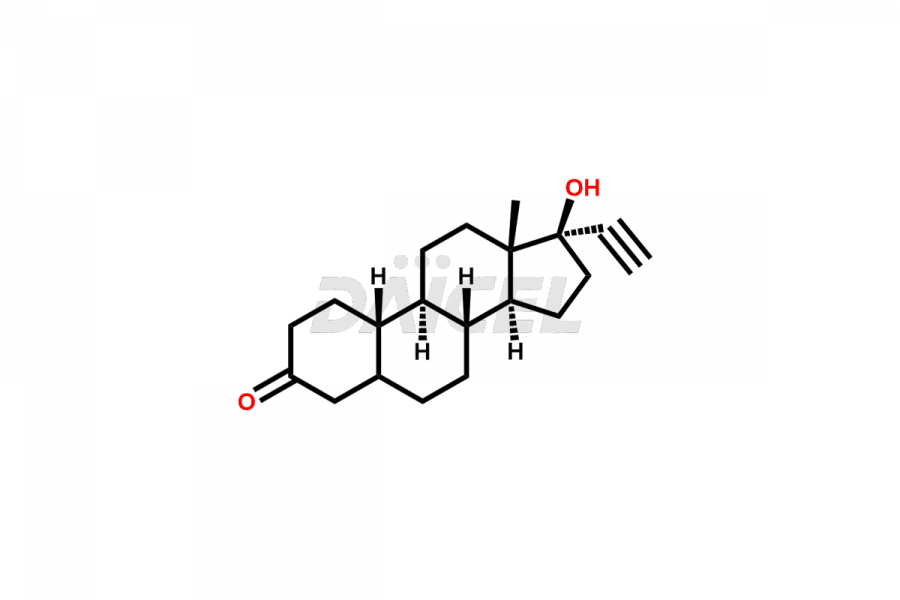
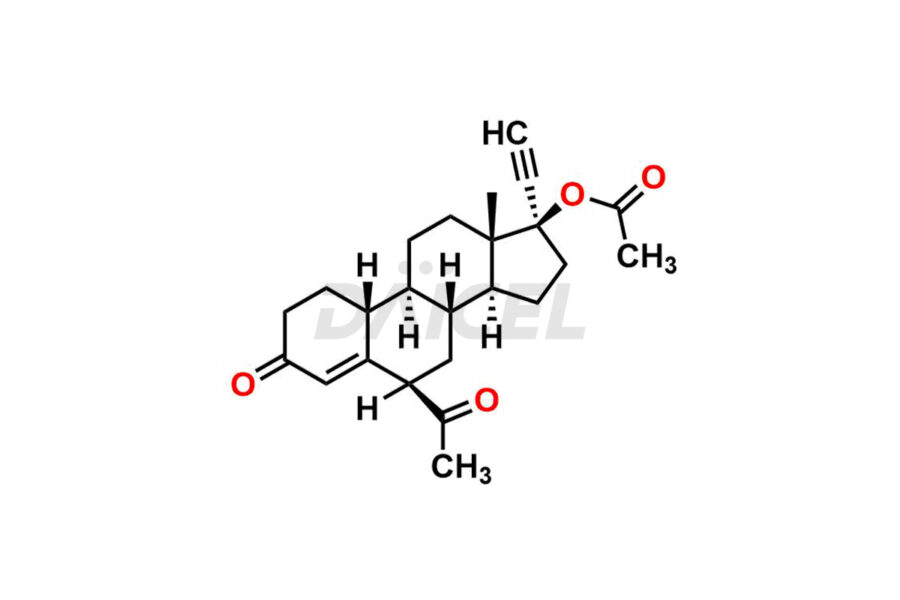
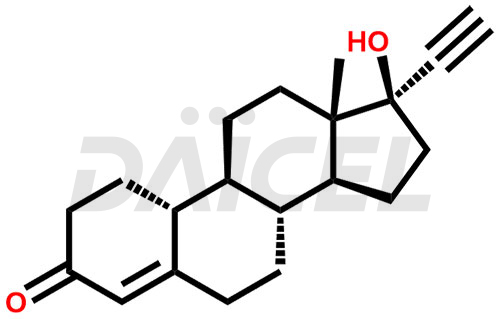
Norethindrone
General Information
Norethindrone Impurities and Norethindrone
Daicel Pharma offers Norethindrone impurities like 6-Hydroxy Norethindrone Acetate, Dihydro Norethindrone (Mixture of Isomers), and Norethindrone Acetate Impurity-D. These impurities are essential for evaluating the quality, stability, and safety of Norethindrone, which is an active pharmaceutical ingredient. Daicel Pharma provides custom synthesis of Norethindrone impurities and distributes them internationally to fulfill clients’ needs.
Norethindrone [CAS: 68-22-4] is a synthetic progestin used for contraception, treating amenorrhea (absence of menstrual periods), and management of endometriosis.
Norethindrone: Use and Commercial Availability
Norethisterone is approved as an oral contraceptive when used alone or with an estrogen component. It helps in hormone replacement therapy in postmenopausal osteoporosis and vasomotor symptoms associated with menopause. Additionally, it is suitable for treating secondary amenorrhea, endometriosis, and hormonal imbalance-related abnormal uterine bleeding. Norethisterone is available under Camila, Emzahh, Errin, Incassia, Micronor, etc.
Norethindrone Structure and Mechanism of Action 
The chemical name of Norethindrone is (17α)-17-Hydroxy-19-norpregn-4-en-20-yn-3-one. Its chemical formula is C20H26O2, and its molecular weight is approximately 298.4 g/mol.
Norethindrone alters cervical mucosa so that it inhibits sperm migration into the uterus. It prevents conception by suppressing ovulation.
Norethindrone Impurities and Synthesis
Norethindrone may contain impurity standards like 6-Hydroxy Norethindrone Acetate, Dihydro Norethindrone (Mixture of Isomers), and Norethindrone Acetate Impurity-D that can arise during the synthesis process or storage. Controlling and monitoring impurities is essential to ensure Norethindrone quality, safety, and efficacy. Stringent quality control measures help meet regulatory standards and ensure the purity of the final product.
Daicel provides a Certificate of Analysis (CoA) of Norethindrone impurity standards. Our cGMP-compliant analytical laboratory offers the CoA, which contains extensive characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity1,2. We give additional characterization data, such as 13C-DEPT, on request. At Daicel Pharma, we can generate unknown impurities of Norethindrone or its degradation products.
References
FAQ's
References
- Braselton, W. E.; Lin, T. J.; Mills, T. M.; Ellegood, J. O.; Mahesh, V. B., Identification and measurement by gas chromatography-mass spectrometry of norethindrone and metabolites in human urine and blood, Journal of Steroid Biochemistry, Volume: 8, Issue: 1, Pages: 9-18, 1977
- Swynnerton, Nollie F.; Fischer, Joseph B., Determination of ethynylestradiol and norethindrone in synthetic intestinal fluid and in timed-release oral formulations, Journal of Liquid Chromatography, Volume: 3, Issue: 8, Pages: 1195-204, 1980
Frequently Asked Questions
Why is it essential to control and monitor Impurities in Norethindrone?
The control and monitoring of Norethindrone impurities assure drug purity, safety, and efficacy. They can change the chemical characteristics of the drug, its stability, or its toxicity profile. They can reduce drug efficacy and pose dangers to human health. Implementing quality control procedures aids in reducing impurities and ensuring the integrity of Norethindrone.
Can Norethindrone Impurities affect its effectiveness?
Impurities in Norethindrone can affect its effectiveness by altering drug pharmacokinetics, pharmacodynamics, or stability. It is crucial to control and monitor impurities to ensure the drug maintains its therapeutic potency and delivers the desired outcome.
Are Norethindrone Impurities harmful?
The impurities do not necessarily imply harm, but their levels and nature need careful regulation. Health authorities set specific limits for impurity levels based on safety and efficacy considerations.
What are the temperature conditions required to store Norethindrone Impurities?
Norethindrone impurities are stored at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.