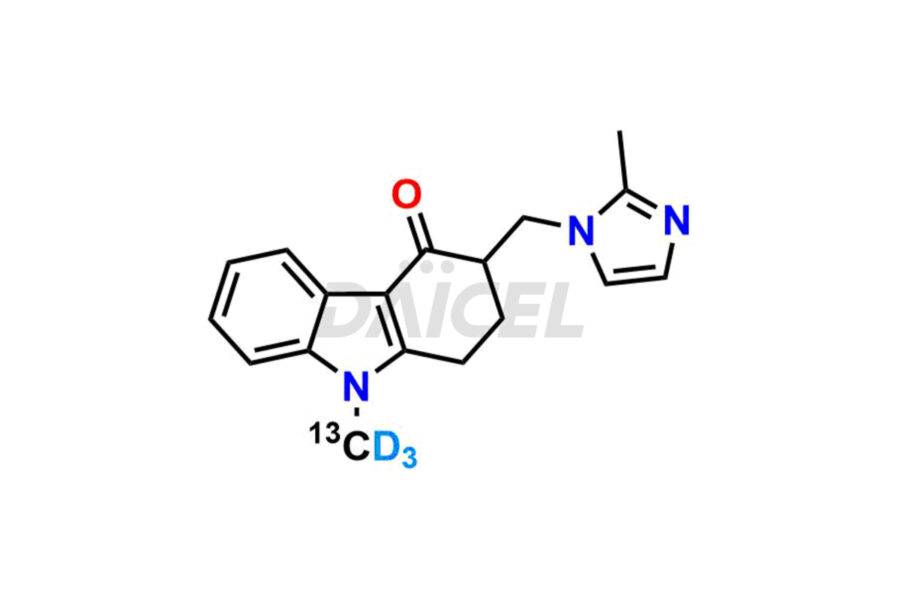
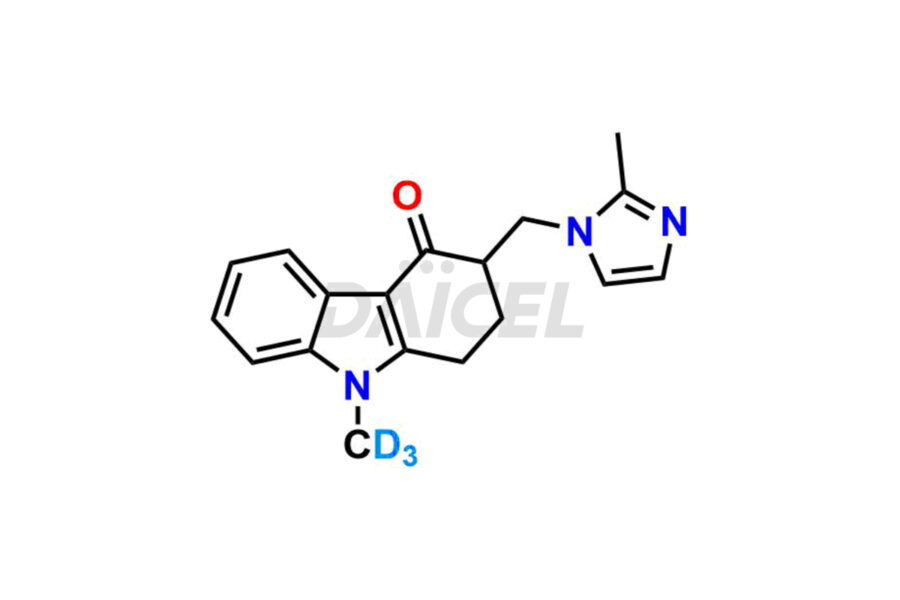
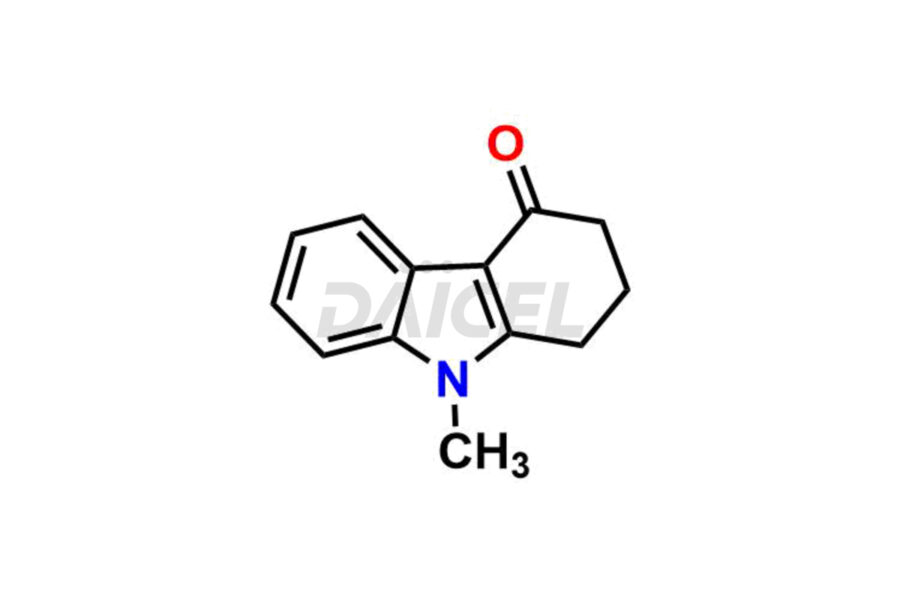
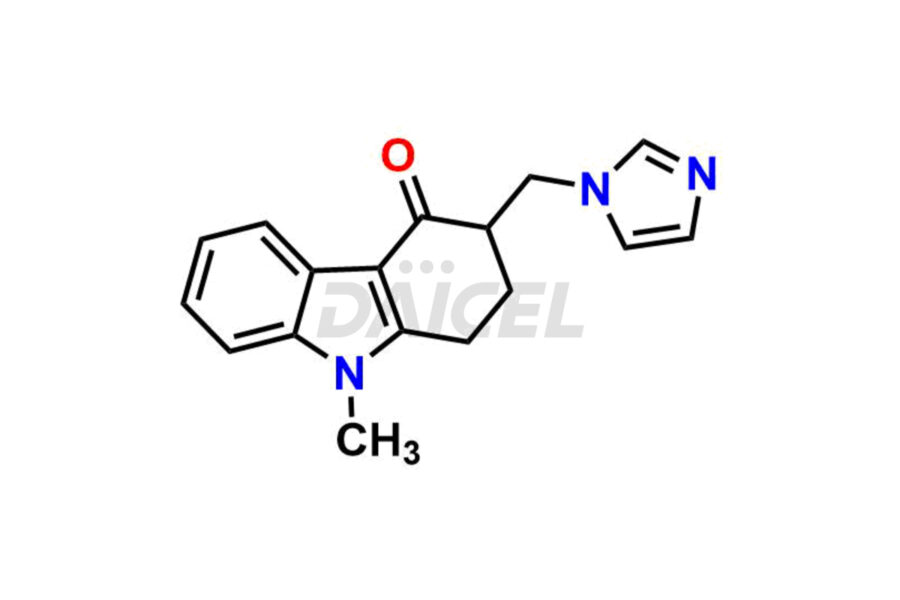
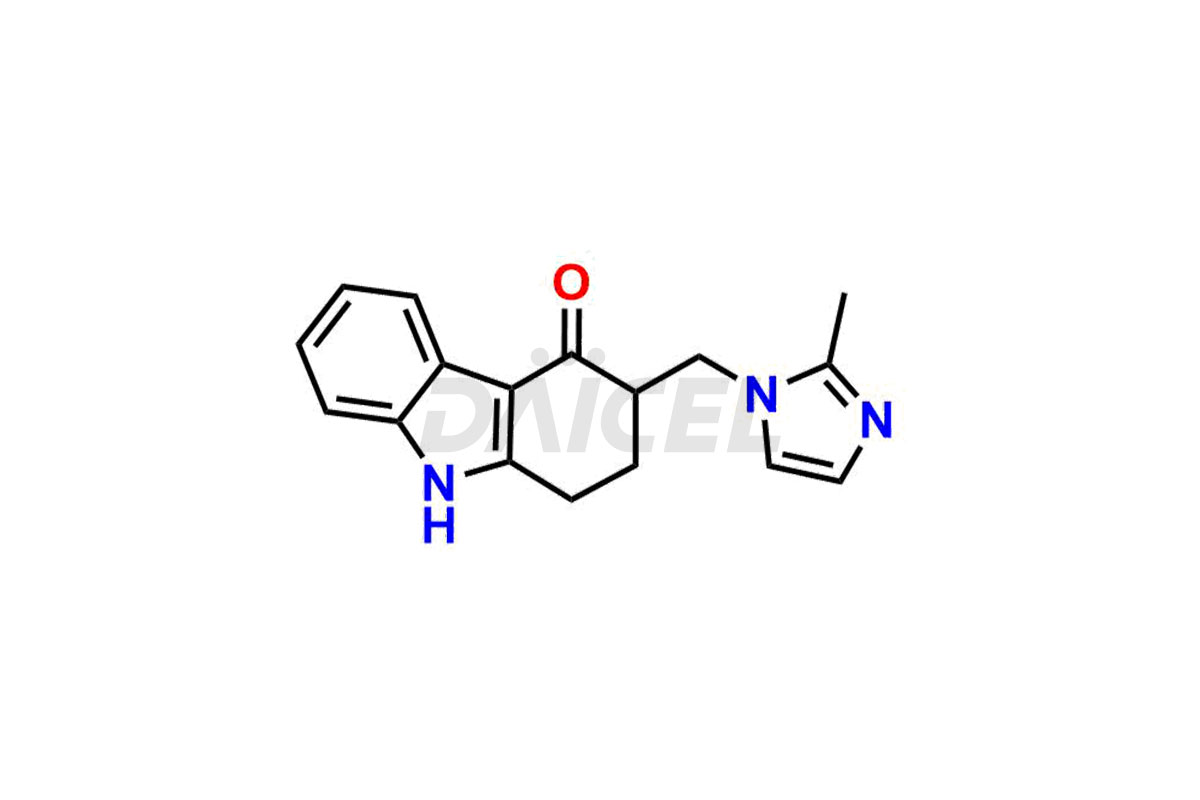
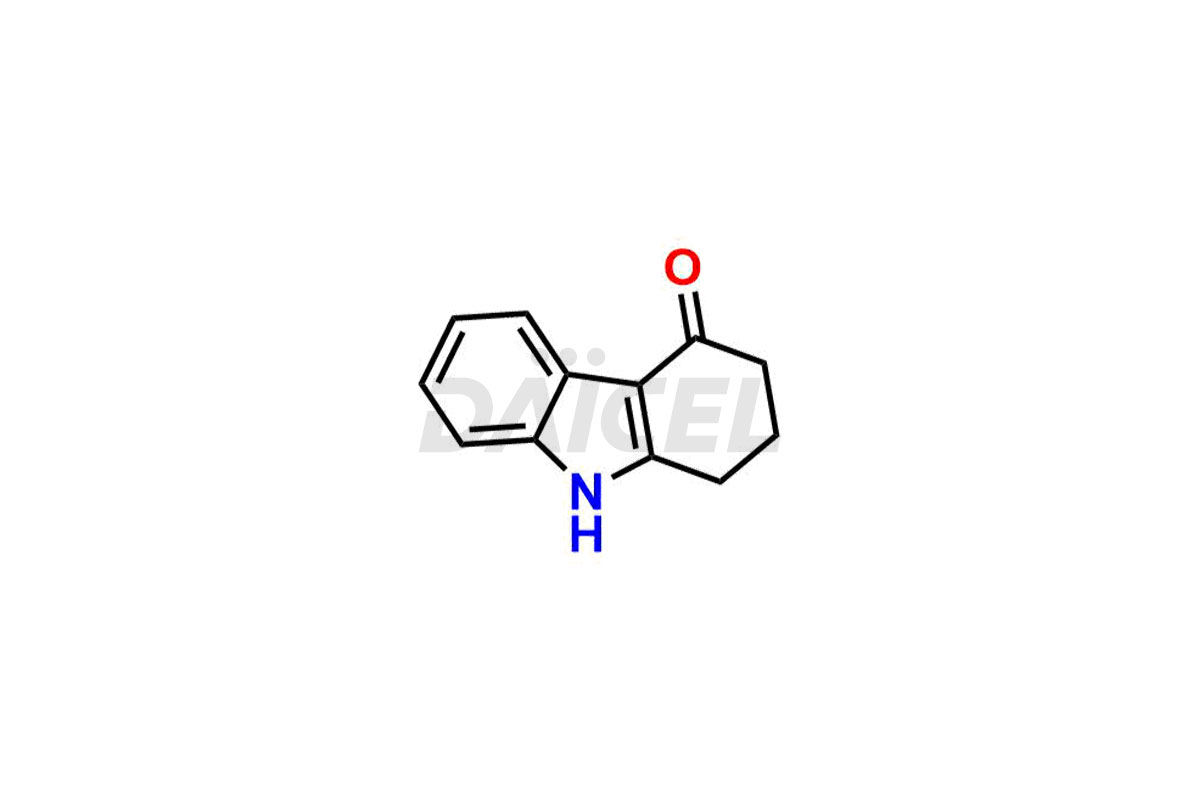
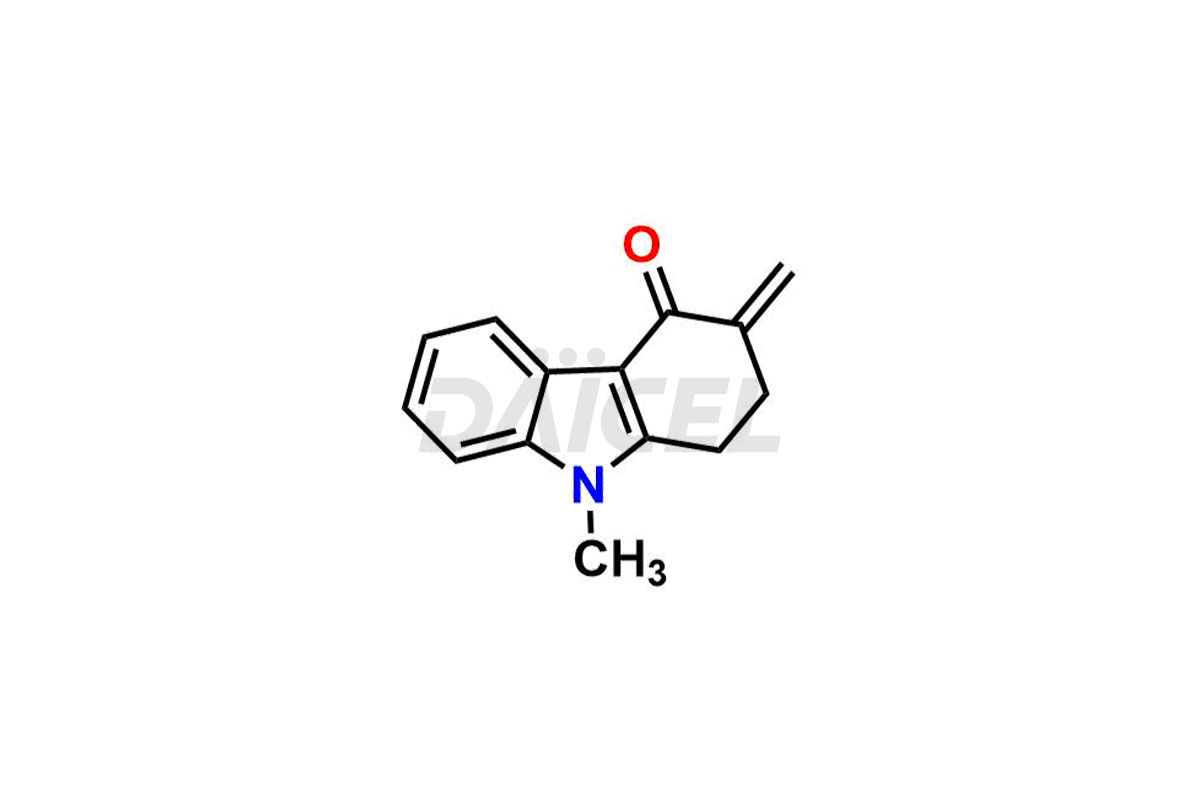
Ondansetron
General Information
Ondansetron Impurities and Ondansetron
Daicel Pharma offers Ondansetron impurities that involve Ondansetron impurity-C, Ondansetron Impurity-G, Ondansetron Impurity-H, Ondansetron Impurity-I, Ondansetron Related Compound A, Ondansetron Related compound – D. These impurities have a role in determining the quality, stability, and safety of Ondansetron, a pharmaceutically active component. Daicel Pharma is capable of synthesizing Ondansetron impurities and distributing them worldwide.
Ondansetron [CAS: 99614-02-5] works as a serotonin type-3 antagonist for treating nausea and vomiting.
Ondansetron: Use and Commercial Availability
Ondansetron reduces nausea and vomiting caused by emetogenic cancer treatment, postoperative care, and radiation. It helps to alleviate postoperative nausea and vomiting. Ondansetron is a frequently used medication for children and teens following head trauma and concussion. Ondansetron comes in different dosage forms, including oral pills, disintegrating tablets, and injectables. It is widely available commercially under several brand names, Zofran and Zuplenz.
Ondansetron Structure and Mechanism of Action
The chemical name of Ondansetron is 1,2,3,9-Tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-4H-carbazol-4-one. Its chemical formula is C18H19N3O, and its molecular weight is approximately 293.4 g/mol.
Its mechanism of action is not known.
Ondansetron Impurities and Synthesis
During the synthesis of Ondansetron impurities, a medication commonly used to prevent nausea and vomiting, impurities can form. These impurities may arise from incomplete reactions, undesired side reactions, or contaminants in the starting materials or reagents. The synthesis1 of Ondansetron involves multiple steps, including reacting various intermediates to form the final product.
Daicel provides a Certificate of Analysis (CoA) of Ondansetron impurity standards such as Ondansetron impurity-C, Ondansetron Impurity-G, Ondansetron Impurity-H, Ondansetron Impurity-I, Ondansetron Related Compound A, and Ondansetron Related compound – D. Daicel delivers a Certificate of Analysis (CoA) from a cGMP-certified analytical facility for Ondansetron impurities. Our CoA contains extensive characterization information, including 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional characterization information, including 13C-DEPT, on request. Our specialists provide the extremely pure Ondansetron D3, Ondansetron 13CD3, deuterated-labeled standards of Ondansetron needed for bioanalytical research, and Bioavailability/Bioequivalence (BA/BE) studies.
References
FAQ's
References
- Coates, Ian Harold; Bell, James Angus; Humber, David Cedric; Ewan, George Blanch, 3-Imidazolylmethyl-1,2,3,9-Tetrahydro-4H-Carbazol-4-One Derivatives, Glaxo Group Ltd., United Kingdom, GB2153821B, August 29, 1985
- Venkateshwaran, T. G.; Stewart, J. T.; King, D. T., HPLC determinations of ondansetron with selected medications in 0.9% sodium chloride injection USP, Journal of Liquid Chromatography & Related Technologies, Volume: 19, Issue: 20, Pages: 3355-3367, 1996
Frequently Asked Questions
Can impurities in Ondansetron be eliminated?
For any pharmaceutical product, including Ondansetron, removing all contaminants is difficult. However, manufacturers reduce impurities using improved synthesis procedures, purification methods, and strict quality control. Impurity levels must be below the permitted limits established by regulatory bodies to guarantee the safety and effectiveness of Ondansetron.
What is the significance of analyzing Ondansetron impurities?
It is vital to ensure drug safety, effectiveness, and quality by identifying, measuring, and regulating undesirable chemicals that may harm patients or interfere with Ondansetron's intended therapeutic activity.
How are Ondansetron impurities synthesized?
Ondansetron impurities are synthesized through various mechanisms during manufacturing, storage, and degradation, arising from incomplete reactions, undesired side reactions, or contaminants in starting materials. Controlling and minimizing them is vital for ensuring Ondansetron's quality and purity.
What are the temperature conditions required to store Ondansetron impurities?
Ondansetron Impurities are stored at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.