LOAD MORE
You're viewed 9 of 41 products
Daicel Pharma synthesizes high-quality Oseltamivir chiral isomers, (3R,4R,5R)- Oseltamivir hydrochloride, (3R,4S,5R)- Oseltamivir hydrochloride, ( 3S,4S,5S)-Oseltamivir hydrochloride, (3S,4R,5S)-Oseltamivir hydrochloride, (3S,4R,5R)-Oseltamivir hydrochloride, (3S,4S,5R)-Oseltamivir hydrochloride (3R,4S,5S)-Oseltamivir hydrochloride. And other impurities like 1,2-Dihydro-Oseltamivir, Oseltamivir Carboxylic Acid, and more, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Oseltamivir. Moreover, Daicel Pharma offers custom synthesis of Oseltamivir impurities and delivers them globally.
Oseltamivir [CAS: 196618-13-0] is a synthetic antiviral prodrug converted to its active form in the liver for treating and preventing influenza A and B. It is a cyclohexene carboxylate ester derived from Oseltamivir acid, which slows down the spread of the influenza virus.
Oseltamivir is a medicine that treats acute and uncomplicated cases of influenza A or B illness in patients having symptoms for not more than two days. It is available under the trade names Oseltamivir Phosphate and Tamiflu.
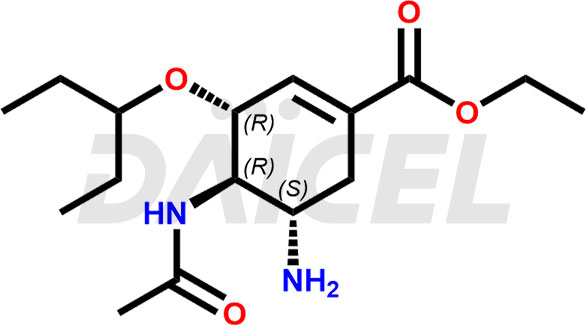
The chemical name of Oseltamivir is (3R,4R,5S)-4-acetylamino-5-amino-3(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid ethyl ester. Its chemical formula is C16H28N2O4, and its molecular weight is approximately 312.40 g/mol.
Oseltamivir inhibits influenza virus neuraminidase altering virus particle aggregation and release. It is an ethyl ester prodrug needing ester hydrolysis for conversion to oseltamivir carboxylate, its active form.
Oseltamivir impurities form during the synthetic process1 or storage of the drug. storage of the drug. They are related to hydrolysis, oxidation, and starting materials or reagents used in the process. These impurities can affect the potency and safety of the drug, so their levels need monitoring and control to ensure the quality of the final product.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Oseltamivir impurity standards high-quality Oseltamivir chiral isomers, (3R,4R,5R)- Oseltamivir hydrochloride, (3R,4S,5R)- Oseltamivir hydrochloride, (3S,4S,5S)-Oseltamivir hydrochloride, (3S,4R,5S)-Oseltamivir hydrochloride, (3S,4R,5R)-Oseltamivir hydrochloride, (3S,4S,5R)-Oseltamivir hydrochloride (3R,4S,5S)-Oseltamivir hydrochloride. And other impurities like 1,2-Dihydro-Oseltamivir, Oseltamivir Carboxylic Acid, and more. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We also provide 13C-DEPT and CHN on request. We give a complete characterization report on delivery.
Daicel has the technology and expertise to prepare any unknown Oseltamivir impurity or degradation product. We also provide labeled compounds to quantify the efficacy of generic Oseltamivir. Daicel offers Oseltamivir -D5, Oseltamivir Carboxylic acid – D3 HCl, and Oseltamivir Hydrochloride-D3, deuterium labeled Oseltamivir compounds used in bio-analytical research, such as BA/BE studies.
Reverse-phase high-performance liquid chromatography method helps detect and analyze the degradation impurities in Oseltamivir.
Impurities in Oseltamivir can affect the drug’s efficacy by decreasing its potency or interfering with its mechanism of action.
Acetonitrile or Methanol are the solvents used in analyzing many Oseltamivir impurities.
Oseltamivir impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.