Ozanimod
General Information
Ozanimod Impurities and Ozanimod
Daicel Pharma offers superior-quality Ozanimod impurities, such as Ozanimod R Isomer. It is vital for evaluating Ozanimod quality, stability, and biological safety. In addition, Daicel Pharma specializes in the custom synthesis of Ozanimod impurities and ensures their worldwide delivery.
Ozanimod [CAS: 1306760-87-1], or RPC10631, is an agonist of sphingosine-1-phosphate receptor-1 (S1P1). It treats relapsing multiple sclerosis in adult patients. In addition, Ozanimod treats ulcerative colitis in patients with inadequate or intolerant responses to conventional medicines.
Ozanimod: Use and Commercial Availability
Zeposia is the brand name of Ozanimod. It is administered orally to patients having multiple sclerosis (MS), relapsing forms. It includes active secondary progressive disease, relapsing-remitting disease, and clinically isolated syndrome. As an Immunomodulator drug, Ozanimod treats moderate to severe ulcerative colitis in adult patients.
Ozanimod Structure and Mechanism of Action

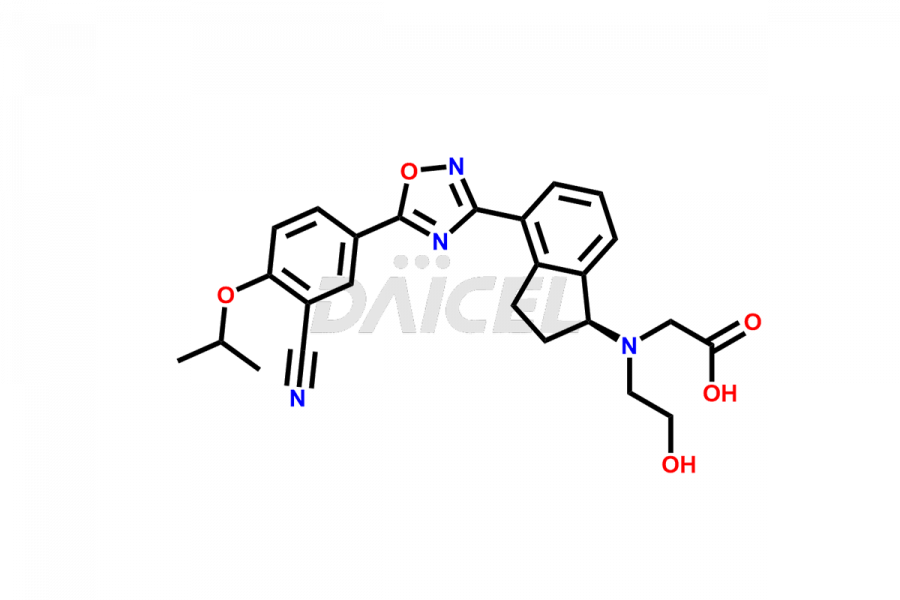
The chemical name of Ozanimod is 5-[3-[(1S)-2,3-Dihydro-1-[(2-hydroxyethyl)amino]-1H-inden-4-yl]-1,2,4-oxadiazol-5-yl]-2-(1-methylethoxy)benzonitrile. The chemical formula for Ozanimod is C23H24N4O3 and its molecular weight is approximately 404.46 g/mol.
As a sphingosine 1-phosphate (S1P) receptor modulator, Ozanimod binds to receptors 1 and 5. It reduces lymphocyte movements from the lymph nodes and targets inflammation sites.
Ozanimod Impurities and Synthesis
During the synthesis of Ozanimod 2, impurities may form that may affect the safety and efficacy of the drug. These impurities form during the synthesis, storage, or degradation of Ozanimod. As a result, Ozanimod impurities must be controlled and monitored throughout the drug’s lifecycle.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Ozanimod impurities, which includes Ozanimod R Isomer. The issued CoA is from a cGMP-compliant analytical facility. It contains detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We give additional data like 13C-DEPT and CHN on request. Daicel Pharma can prepare any unidentified Ozanimod impurity or degradation product. In addition, Daicel Pharma offers highly purified isotope-labeled standards of Ozanimod for bioanalytical research and BA/BE studies. We also provide a complete characterization report on delivery.
References
FAQ's
References
- Scott, F. L.; Clemons, B.; Brooks, J.; Brahmachary, E.; Powell, R.; Dedman, H.; Desale, H. G.; Timony, G. A.; Martinborough, E.; Rosen, H.; et al, Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1) and receptor-5 (S1P5) agonist with autoimmune disease-modifying activity, British Journal of Pharmacology, Volume: 173, Issue: 11, Pages: 1778-1792, 2016 DOI: (10.1111/bph.13476)
- Martinborough, Esther; Boehm, Marcus F.; Yeager, Adam Richard; Tamiya, Junko; Huang, Liming; Brahmachary, Enugurthi; Moorjani, Manisha; Timony, Gregg Alan; Brooks, Jennifer L.; Peach, Robert; et al, Selective sphingosine 1 phosphate receptor modulators and methods of chiral synthesis, WO2011060392A1, May 19, 2011, Receptos, Inc., United States
Frequently Asked Questions
What is the source of the presence of Ozanimod impurities in the drug?
Unreacted starting materials, residual solvents, and intermediates are the source of Ozanimod impurities in the drug.
How are Ozanimod impurities detected and quantified?
Ozanimod impurities are detected and quantified using analytical methods such as HPLC and LC-MS.
Which guidelines control potential Ozanimod impurities?
ICH Q3A and Q3C guidelines control potential Ozanimod impurities.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.