Pacritinib
General Information
Pacritinib Impurities and Pacritinib
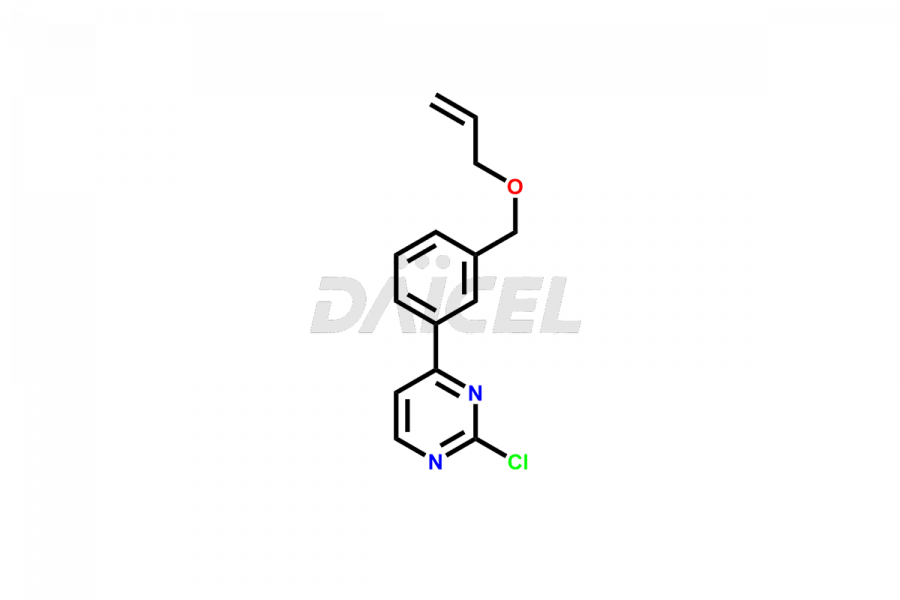
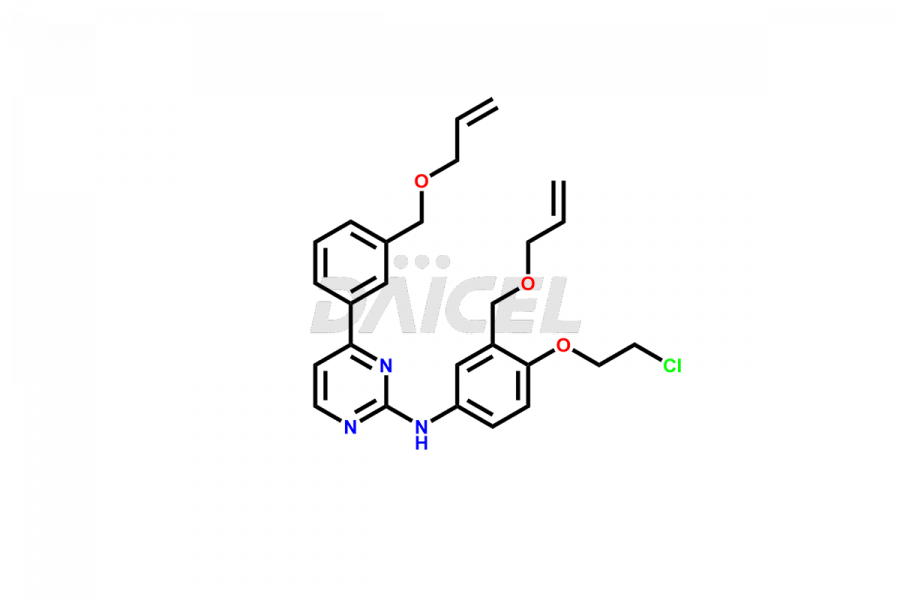
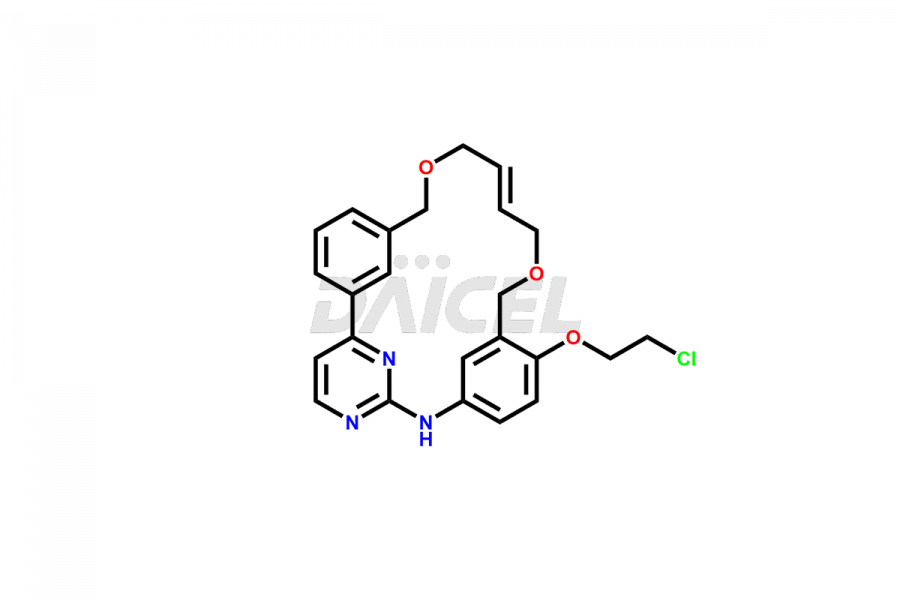
Daicel Pharma offers high-quality Pacritinib impurities, such as 4-(3-((allyloxy)methyl)phenyl)-2-chloropyrimidine, N-(3-((allyloxy)methyl)-4-(2-chloroethoxy)phenyl)-4-(3-((allyloxy)methyl)phenyl)pyrimidin-2-amine, and Pacritinib Chloro Impurity. It is vital for evaluating Pacritinib quality, stability, and biological safety. In addition, Daicel Pharma specializes in the custom synthesis of Pacritinib impurities and ensures their worldwide delivery.
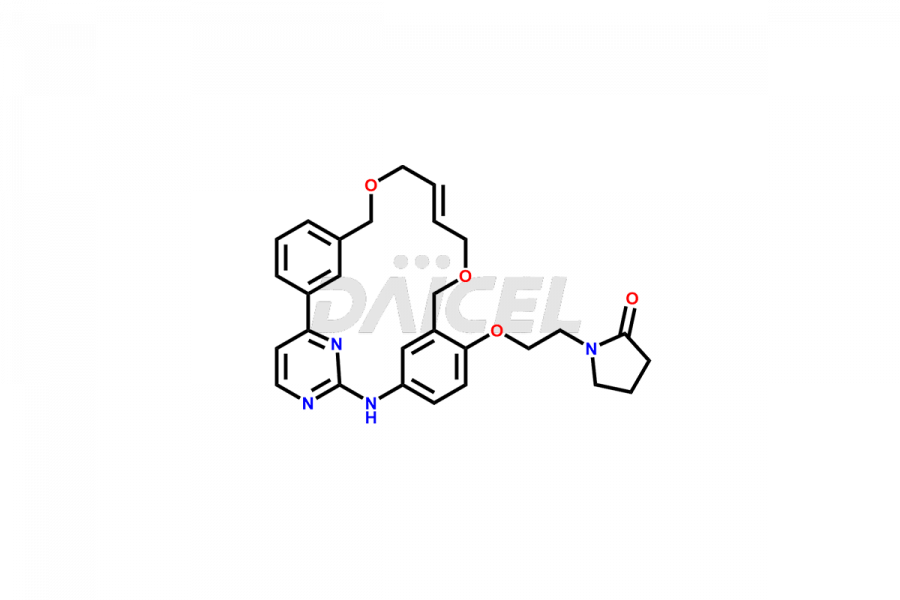
Pacritinib [CAS: 937272-79-2] is an oxygen-linked macromolecule that treats myelofibrosis. Developed by CTI Biopharma, Pacritinib is a JAK2 inhibitor. It treats adults with high-risk or intermediate primary or secondary myelofibrosis. In addition, it blocks FMS-like tyrosine kinase 3 (FLT3), interleukin-1 receptor-associated kinase 1 (IRAK1), and colony-stimulating factor 1 receptor (CSF1R). Pacritinib helps in cancer therapy.
Pacritinib: Use and Commercial Availability
Pacritinib treats a rare myeloproliferative neoplasm, myelofibrosis, with symptoms of splenomegaly and cytopenias. Further, it treats severe thrombocytopenia associated with myelofibrosis. It treats myelofibrosis patients having platelet counts below 50 × 109/L. Pacritinib has demonstrated significant anti-tumor activity in preclinical studies. It is administered orally to patients. Pacritinib is available under the brand name Vonjo.
Pacritinib Structure and Mechanism of Action

The chemical name of Pacritinib is (16E)-11-[2-(1-Pyrrolidinyl)ethoxy]-14,19-dioxa-5,7,27-triazatetracyclo[19.3.1.12,6.18,12]heptacosa-1(25),2,4,6(27),8,10,12(26),16,21,23-decaene. The chemical formula for Pacritinib is C28H32N4O3, and its molecular weight is approximately 472.58 g/mol.
Pacritinib is a kinase inhibitor that acts against Janus-associated kinase2 and mutation V617F in Janus Kinase 2 (JAK2). It also blocks FMS-like tyrosine kinase 3, which signals growth factors and cytokines, useful for immune functions and hematopoiesis.
Pacritinib Impurities and Synthesis
While synthesizing Pacritinib 1,2, impurities may form that affect the safety and efficacy of the drug. During the production and storage of Pacritinib, impurities form. Thus, Pacritinib impurities need to be controlled and monitored at every stage of drug development.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Pacritinib impurities, which includes 4-(3-((allyloxy)methyl)phenyl)-2-chloropyrimidine, N-(3-((allyloxy)methyl)-4-(2-chloroethoxy)phenyl)-4-(3-((allyloxy)methyl)phenyl)pyrimidin-2-amine, and Pacritinib Chloro Impurity. The issued CoA is from a cGMP-compliant analytical facility. It contains detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We give additional data like 13C-DEPT and CHN on request. Daicel Pharma can make any unidentified Pacritinib impurities or degradation products. In addition, Daicel Pharma offers highly purified isotope-labeled standards of Pacritinib for bioanalytical research and BA/BE studies. We also provide a complete characterization report on delivery.
References
FAQ's
References
- Blanchard, Stephanie; Lee, Cheng Hsia Angeline; Nagaraj, Harish Kumar Mysore; Poulsen, Anders; Sun, Eric T.; Tan, Yee Ling Evelyn; William, Anthony Deodaunia, Oxygen linked pyrimidine derivatives, WO2007058627A1, May 24, 2007, S*bio Pte Ltd, Singapore
- William, Anthony D.; Lee, Angeline C.-H.; Blanchard, Stephanie; Poulsen, Anders; Teo, Ee Ling; Nagaraj, Harish; Tan, Evelyn; Chen, Dizhong; Williams, Meredith; Sun, Eric T.; et al, Discovery of the Macrocycle 11-(2-Pyrrolidin-1-yl-ethoxy)-14,19-dioxa-5,7,26-triaza-tetracyclo[19.3.1.1(2,6).1(8,12)]heptacosa-1(25),2(26),3,5,8,10,12(27),16,21,23-decaene (SB1518), a Potent Janus Kinase 2/Fms-Like Tyrosine Kinase-3 (JAK2/FLT3) Inhibitor for the Treatment of Myelofibrosis and Lymphoma, Journal of Medicinal Chemistry, Volume: 54, Issue: 13, Pages: 4638-4658, 2011 DOI: (10.1021/jm200326p)
Frequently Asked Questions
What are the solvents in which Pacritinib impurities are soluble?
DMSO and acetonitrile are the solvents in which Pacritinib impurities are soluble.
What causes the formation of potential genotoxic Pacritinib impurities?
Starting materials and intermediates used or prepared during the manufacturing may be responsible for forming potential genotoxic Pacritinib impurities.
Why is it vital to control and monitor Pacritinib impurities?
The presence of Pacritinib impurities in the drug can affect drug safety, resulting in drug recalls. Hence, it is vital to control and monitor Pacritinib impurities as per regulatory guidelines.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.