LOAD MORE
You're viewed 9 of 16 products
Daicel Pharma specializes in offering Perindopril impurity standards, including Perindopril Impurity, Perindopril impurity B, Perindopril EP impurity G, Perindopril EP impurity H, Perindopril EP impurity F, Perindopril EP Impurity C, Perindopril (S, S, S, R, S) isomer and more. The presence of these impurities can significantly affect the effectiveness, stability, and safety of Perindopril. Daicel Pharma can synthesize and provide custom Perindopril impurities in compliance with international standards and regulations.
Perindopril [CAS: 82834-16-0] is an antihypertensive drug and and a peptidyl-dipeptidase A inhibitor. It treats hypertension and stable coronary artery disease.
Perindopril is a member of the angiotensin-converting enzyme (ACE) inhibitor medication family. Perindopril treats mild to moderate essential hypertension and mild to moderate congestive heart failure and reduces the cardiovascular risk of those having hypertension, a previous heart attack, or stable coronary disease. This drug is available in the market under Aceon.
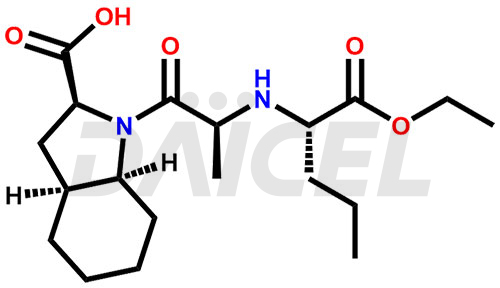
The chemical name of Perindopril is (2S,3aS,7aS)-1-[(2S)-2-[[(1S)-1-(Ethoxycarbonyl)butyl]amino]-1-oxopropyl]octahydro-1H-indole-2-carboxylic acid. Its chemical formula is C19H32N2O5, and its molecular weight is approximately 368.5 g/mol.
Perindopril inhibits ACE activity, decreases vasoconstriction, increases plasma renin activity, and decreases aldosterone secretion.
During the synthesis1 of Perindopril, impurities may form through various pathways. They can arise from incomplete reactions, side reactions, or contaminants in the starting materials or reagents. The specific synthesis routes for Perindopril impurities can vary depending on the particular impurities and the conditions.
Daicel Pharma provides a Certificate of Analysis (CoA) for Perindopril impurity standards, which include Perindopril Impurity, Perindopril impurity B, Perindopril EP impurity G, Perindopril EP impurity H, Perindopril EP impurity F, Perindopril EP impurity D, Perindopril EP Impurity C, Perindopril (S, S, S, R, S) isomer and more. At Daicel Pharma, we provide a Certificate of Analysis (CoA) from our cGMP-certified analytical lab, which includes extensive characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additional characterization information, including 13C-DEPT, can be provided upon request. Daicel Pharma specializes in synthesizing Perindopril impurities and degradation products.
Impurities in Perindopril can form through various routes, including degradation reactions during synthesis, storage, or exposure to environmental factors, as well as impurity introduction from raw materials or by-products of the synthesis process.
Analyzing Perindopril impurities is significant for ensuring safety, efficacy, and quality, as they may affect its effectiveness, stability, and overall safety profile.
Following regulatory rules and standards, Perindopril impurities are discovered and categorized using analytical techniques, including chromatography, spectroscopy, and mass spectrometry, based on their chemical structure, retention duration, and spectral data.
Perindopril impurities are stored at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.