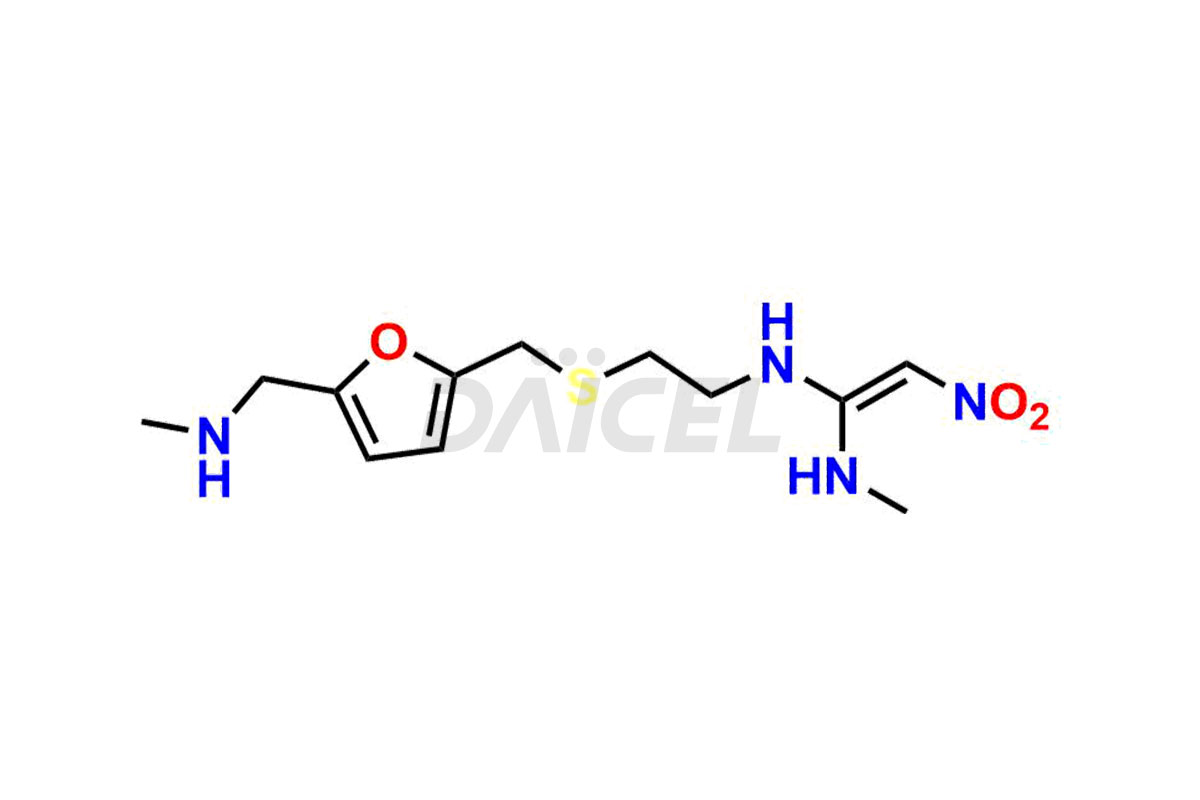
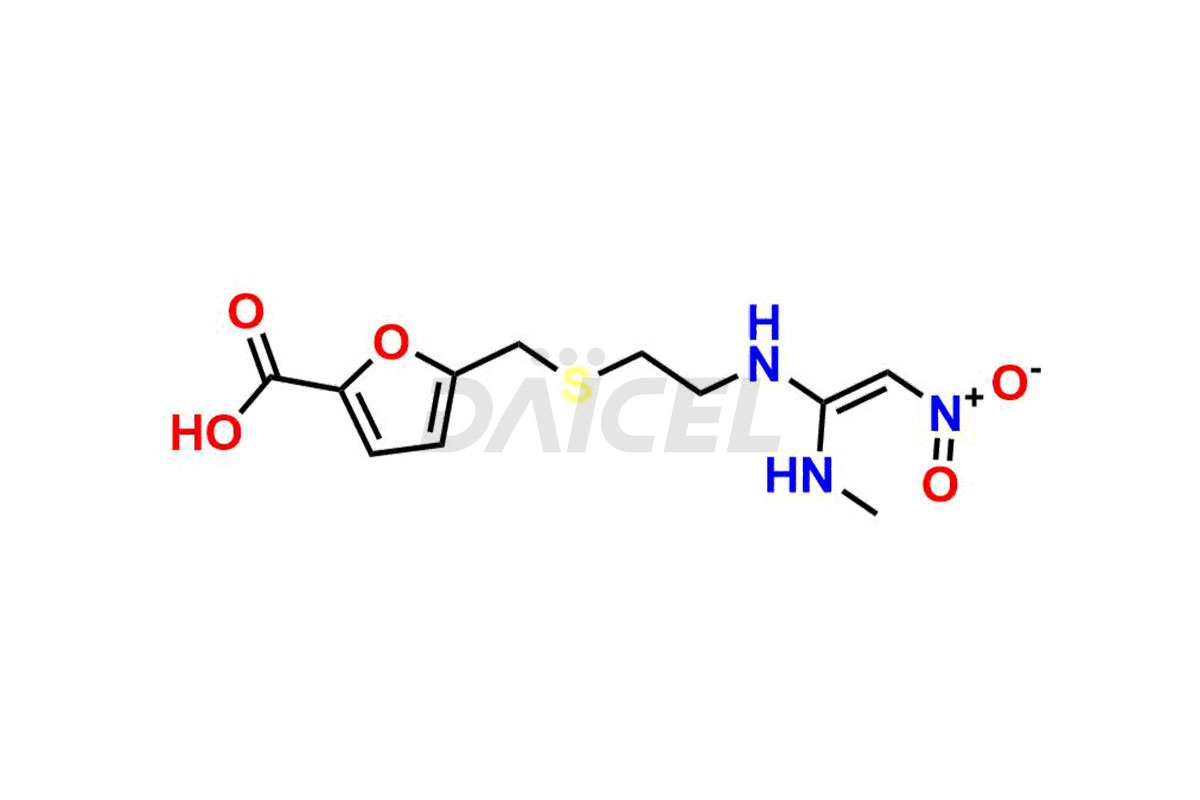
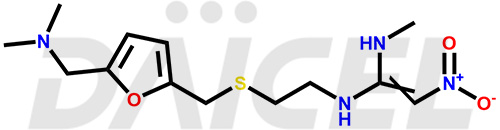
Ranitidine
General Information
Ranitidine Impurities and Ranitidine
Daicel Pharma offers high-quality Ranitidine impurity standards, Ranitidine-furoic acid analogue, and N-Desmethyl Ranitidine. The impurities affect Ranitidine’s effectiveness, stability, safety, and quality. Moreover, Daicel Pharma provides customized Ranitidine impurities and ships them worldwide.
Ranitidine [CAS: 66357-35-5] is a furan derivative and treats gastroesophageal reflux disease (GERD) and peptic ulcer disease (PUD). It functions as an anti-ulcer drug.
Ranitidine: Use and Commercial Availability
Ranitidine is a histamine type 2 receptor antagonist (H2 blocker) that treats acid reflux and heartburn. It is an environmental pollutant, a xenobiotic, and a drug allergen. This drug is available under the brand names Tritec and Zantac.
Ranitidine Structure and Mechanism of Action 
The chemical name of Ranitidine is N-[2-[[[5-[(Dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N′-methyl-2-nitro-1,1-ethenediamine. Its chemical formula is C13H22N4O3S, and its molecular weight is approximately 314.41 g/mol.
Ranitidine inhibits histamine and prevents acid secretion induced by food.
Ranitidine Impurities and Synthesis
Ranitidine impurities are chemical compounds that occur unintentionally during the manufacturing1 or storage of Ranitidine, a drug used to treat illnesses such as ulcers and gastroesophageal reflux disease (GERD). Impurities occur due to starting materials, reagents, intermediates, or degradation products. Ranitidine impurities require strict control and monitoring to assure Ranitidine’s safety, efficacy, and quality, and analytical procedures help identify, quantify, and characterize these impurities.
Daicel Pharma offers a Certificate of Analysis (CoA) for Ranitidine impurity standards, Ranitidine-furoic acid analogue and N-Desmethyl Ranitidine. Our cGMP-compliant analytical facility provides a comprehensive CoA with detailed characterization data like 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additional characterizations like 13C-DEPT are available upon request.
References
FAQ's
References
- Price, Barry John; Clitherow, John Watson; Bradshaw, John, Aminoalkyl Furan Derivatives, Allen and Hanburys Ltd., United Kingdom, GB1565966A, April 23, 1980
- Carey, P. F.; Martin, L. E., A high-performance liquid chromatography method for the determination of ranitidine in plasma, Journal of Liquid Chromatography, Volume: 2, Issue: 9, Pages: 1291-303, 1979
Frequently Asked Questions
How are Ranitidine impurities controlled?
Ranitidine impurities require strict control with quality control procedures. Specific limits for impurity levels imposed by regulatory agencies need monitoring.
Can Ranitidine impurities affect its effectiveness?
Impurities in Ranitidine can reduce its effectiveness by affecting the drug's pharmacokinetics, pharmacodynamics, or stability. They need control and monitoring to ensure the medicine retains its therapeutic potency and achieves the targeted therapeutic effects.
Are generic versions of Ranitidine tested for impurities?
Impurities in Ranitidine generics are rigorously examined to ensure their quality, safety, and efficacy.
What are the temperature conditions required to store Ranitidine impurities?
Ranitidine impurities should be stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.